
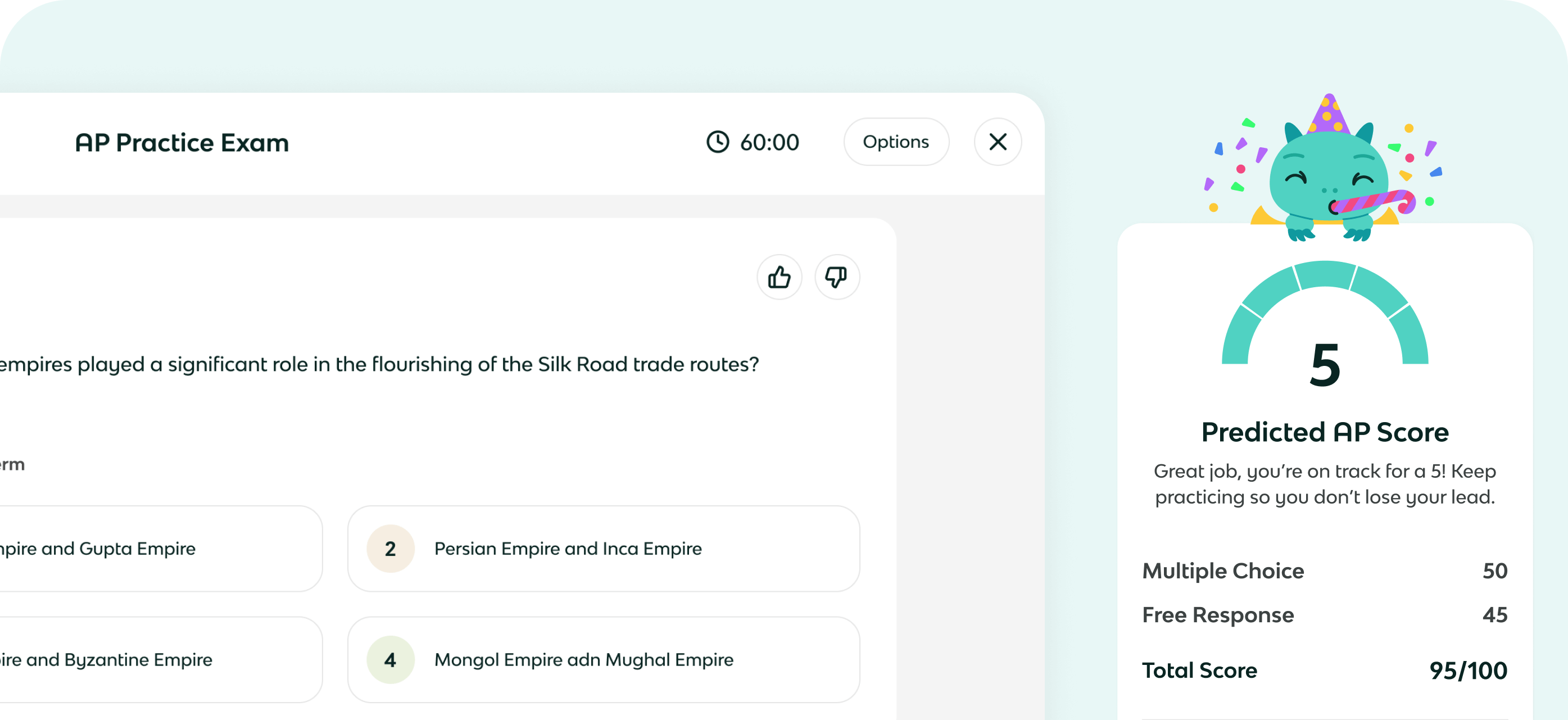
AP Chemistry FRQ Room
Ace the free response questions on your AP Chemistry exam with practice FRQs graded by Kai. Choose your subject below.
Knowt can make mistakes. Consider checking important information.
AP Chemistry Free Response Questions
The best way to get better at FRQs is practice. Browse through dozens of practice AP Chemistry FRQs to get ready for the big day.
Analyzing Ion Concentrations in Environmental Samples
Environmental geochemistry: Analyze a sample of freshwater with a measured sodium ion concentration.
Application of the Ideal Gas Law: Gas Evolution Reaction
A chemical reaction conducted in a closed system produces a gas that is collected at Standard Temper
Comprehensive Analysis on Periodic Trends and Chemical Properties
Synthesize your understanding of periodic trends and design an experiment linking atomic size and io
Coulomb's Law in Electron Binding Energy Measurement Flaw
A physics student conducts an experiment to measure the binding energy of an electron in a hydrogen-
Determining Avogadro's Number Using Brownian Motion
Avogadro's number can be estimated through the analysis of Brownian motion in a colloidal suspension
Determining Empirical and Molecular Formulas from Percent Composition
An unknown compound was analyzed and its percent composition is given in the table below. Use the da
Determining the Molar Mass of an Unknown Gas at STP via the Ideal Gas Law
Using the ideal gas law at Standard Temperature and Pressure (STP), design an experiment to determin
Effect of Electron Configuration on Chemical Bonding Patterns
This question examines how electron configuration influences the formation and characteristics of di
Electron Configuration Determination from Spectroscopic Data
A spectroscopic study of unknown elements reveals several emission lines corresponding to electron t
Electronegativity and Bond Polarity
Consider a carbon-oxygen bond. Given the electronegativity of carbon is 2.55 and that of oxygen is 3
Electronegativity and Bond Polarity
Bond polarity arises from differences in electronegativity. Answer the following:
Electrostatic Attraction and Atomic Energy Levels
Discuss the role of Coulomb's law in atomic interactions and link it to discrete energy levels obser
Empirical and Molecular Formula Determination
A laboratory has analyzed a compound and determined its percent composition. The analysis indicates
Graphical Analysis of a Photoelectron Spectrum with Misinterpreted Peak Assignments
A student analyzes a photoelectron spectrum of a main-group element by plotting relative intensity v
Ideal Gas Law and Mole Determination
An unknown gas is studied at STP. The following experimental data were recorded: Volume = $$22.4$$ L
Isotopic Abundance Analysis for Selenium
A mass spectrum of selenium shows several peaks corresponding to its naturally occurring isotopes. U
Mass Spectrum Analysis of Selenium Isotopes
A mass spectrum for selenium shows data for its naturally occurring isotopes as given in the table.
Percent Composition and Stoichiometry
In an environmental analysis, a compound is found in a mineral with a mass composition of 40% metal
Percent Composition of Calcium Nitrate
You will calculate the percent composition by mass for the compound calcium nitrate, Ca(NO₃)₂.
Periodic Table and Isotopic Composition Analysis
In this question, analyze the information provided by the periodic table regarding element carbon an
Predicting Ionic Charges and Compound Formation
Using periodic trends, predict ionic charges and determine the formulas of compounds. Answer the fol
Advanced Ionic Compound Lattice Energy Analysis: A Comparative Study
In this question, you will derive the relationship between ionic charges, interionic distance, and l
Bond Order, Bond Length, and Experimental Correlations
This question explores the theoretical relationship between bond order, bond length, and bond energy
Comparative Analysis of Bond Types in Materials
A materials scientist is designing a new composite material by integrating different types of bondin
Comprehensive Lewis Structure and Formal Charge Analysis in Sulfate Ion
This question requires you to integrate several concepts in Lewis structure construction, resonance,
Conductivity and Phase Change in Ionic Substances
Electrical conductivity varies with the phase of the substance. Using the conductivity table provide
Conductivity in Ionic and Molecular Substances
A laboratory experiment analyzes the electrical conductivity of various substances in different phas
Critique of a Potential Energy Diagram
A potential energy diagram for a covalent bond is provided but may contain errors or mislabeling. An
Determination of Bonding Type through Conductivity Experiments
An experiment measures the electrical conductivity of substances in their molten state. The substanc
Determining Molecular Polarity and Intermolecular Forces
This question investigates how molecular geometry and electronegativity differences lead to polarity
Effect of Expanded Octets on Molecular Structure: $$\text{PCl}_5$$ and $$\text{SF}_6$$
This question explores the concept of expanded octets by requiring drawing Lewis structures for $$\t
Effect of Ion Size and Charge Density on Bond Strength
Use Coulomb's law to analyze how differences in ion size and charge density affect the strength of i
Expanded Octets: Analysis of Sulfur Hexafluoride
Sulfur hexafluoride ($$SF_{6}$$) is an example of a compound in which the central atom has an expand
Experimental Claims on Alloy Conductivity
A media report suggests that introducing a minor percentage of a secondary metal into a primary meta
Experimental Design: Identifying Bonding Types via Conductivity
Design an experiment that uses conductivity measurements to determine the bonding type of an unknown
Formal Charge Analysis in Nitrite Ion
Examine the nitrite ion $$NO_{2}^{-}$$ by considering its possible Lewis dot structures.
FRQ12: Bond Polarity and Its Impact on Molecular Properties
Bond polarity, arising from differences in electronegativity, plays a major role in determining a mo
FRQ13: Quantitative Analysis of Coulomb's Law in Ionic Crystals
A theoretical study is conducted where students calculate ionic bond energies using Coulomb’s law. G
FRQ19: Impact of Ionic Radii on Lattice Structures
The lattice structure of ionic compounds depends significantly on the sizes of the constituent ions.
Hybridization, Lone Pairs, and Molecular Geometry of SF₄
The molecule SF₄ exhibits unique molecular geometry due to its electron pair arrangement. Analyze it
Interpreting Metallic Bonding Representations
Metallic bonding is characterized by a lattice of positive ions immersed in a 'sea of electrons'. An
Ionic Size Effects on Lattice Energy
The lattice energy of ionic compounds is influenced by both the charge of the ions and their ionic r
Kinetic Analysis in Gas-Phase Reactions with Collisional Theory Error
A researcher examines the kinetics of a gas-phase reaction involving a covalent molecule by measurin
Melting Point Investigation in Ionic Solids
A researcher studies the melting points of various ionic solids to understand the factors that influ
Metallic Bonding and Alloy Formation
Investigate the unique characteristics of metallic bonding and alloy formation, focusing on how the
Molecular Geometry of Sulfur Tetrafluoride (SF4)
A researcher synthesizes sulfur tetrafluoride ($$\text{SF}_4$$) and seeks to understand its bonding
Phase Transitions and Conductivity of Ionic Compounds
A physical chemist is examining how the electrical conductivity of an ionic compound changes as it t
Potential Energy Curves and Bond Orders
A physical chemist examines a potential energy curve for a diatomic molecule to understand the relat
Resonance and Bond Order Calculation
This question focuses on the effect of resonance on bond characteristics, particularly bond order, a
Resonance and Uniformity of Bond Lengths in Aromatic Compounds
Resonance in aromatic compounds such as benzene leads to uniform bond lengths. Analyze how resonance
Temperature Effects on Equilibrium in Covalent Network Solids
A researcher attempts to investigate the effect of temperature on the equilibrium of bonds within si
UV-Vis Analysis of Conjugated Molecules and Electron Delocalization
A researcher analyzes a series of conjugated organic molecules using UV-Vis spectroscopy to assess t
Analysis of Ideal Gas Law Behavior under Varying Conditions
Examine the experimental conditions provided in the data table and analyze how pressure, volume, and
Beer’s Law and Concentration Measurement
A solution containing a colored compound is analyzed using a spectrophotometer. The measured absorba
Beer’s Law in Quantitative Analysis
Investigate the application of Beer’s Law for determining solute concentration in a solution.
Bond Polarity in NH3 vs. PH3
Compare and contrast the bond polarity and overall molecular polarity in ammonia (NH3) and phosphine
Chromatography Data Analysis for Ink Separation
Analyze the chromatographic separation of ink components to determine the polarity of the pigments.
Chromatography: Molecule Polarity and Rf Values
Chromatography data can be used to infer the polarity of substances. Analyze the experiment using pr
Combined Gas Law Application
A sample of gas initially has a pressure $$P_{1}= 2\ \text{atm}$$, a volume $$V_{1}= 10\ \text{L}$$,
Comprehensive Analysis of Gaseous Systems
A researcher conducts a comprehensive study of a gaseous system by integrating kinetic molecular the
Determination of Molar Mass from Gas Density
It is possible to determine the molar mass of a gas from its measured density. Derive the expression
Determination of Partial Pressures Using Dalton's Law
Atmospheric scientists study the composition of air by analyzing partial pressures. A sealed contain
Deviation from Ideal Behavior at High Pressures
When gases are compressed to very high pressures, deviations from ideal gas behavior become signific
Distillation and Phase Changes
A researcher employs fractional distillation to separate a mixture of two liquids with different boi
Effusion and Molar Mass Calculation
A researcher determines the molar mass of an unknown gas by measuring its rate of effusion compared
Electromagnetic Spectrum and Electron Transitions
A researcher is investigating how molecules interact with electromagnetic radiation during electroni
Electronic Transitions and the Electromagnetic Spectrum
Investigate how electronic transitions in atoms lead to photon absorption and the resulting spectral
Energy Changes and Spectroscopy
Connect the quantized energy changes in atoms to spectral observations in a laboratory setting.
Energy Transitions and the Electromagnetic Spectrum
Evaluate the relationship between wavelength and the type of energy transition in atoms using the pr
Gas Density and Molar Mass Determination
This question involves deriving a relationship between a gas's density and its molar mass, and then
Gas Effusion and Molar Mass Determination
Utilize Graham’s Law to determine the molar mass of an unknown gas based on its effusion rate compar
Gas Mixtures, Reaction Rates, and Kinetic Theory
During a combustion reaction, the reaction rate is influenced by the kinetic energy of gas molecules
High Pressure Gas Behavior
Real gases deviate from ideal behavior under high pressure due to the finite volume of molecules and
Hydrogen Bonding in Water vs. Ammonia
A researcher investigates the role of hydrogen bonding in determining the physical properties of wat
Intermolecular Forces and Boiling Points
Intermolecular forces (IMFs) determine physical properties such as boiling points. Analyze the relat
Intermolecular Forces Ranking in Different Substances
Consider four substances: iodine (I2), water (H2O), oxygen (O2), and sodium chloride (NaCl).
Maxwell-Boltzmann and Kinetic Energy Analysis
Utilize the Maxwell-Boltzmann graph to analyze the effects of temperature and molecular mass on the
Non-Ideal Gas Behavior Analysis
Compare experimental gas pressures with predictions from the ideal gas law and explain the factors c
Phase Changes and Bond Energy Considerations
A chemist is investigating the energy requirements for phase transitions in substances characterized
Solubility Analysis of Polar and Nonpolar Compounds
A student aims to compare the solubility of polar and nonpolar compounds by dissolving equal masses
Spectrophotometric Determination of Molecular Polarity
A student conducts an IR spectroscopy experiment to assess the polarity of various organic compounds
Spectroscopy: Electronic Transitions
A molecule’s absorption spectrum is used to study its electronic transitions. Answer the following:
Acid-Base Equilibria and Conjugate Pairs
This FRQ examines acid-base equilibria using acetic acid as an example. The dissociation reaction is
Acid-Base Equilibrium of Acetic Acid
Acetic acid reacts with water according to the equilibrium: $$HC_2H_3O_2 + H_2O \rightleftharpoons C
Acid-Base Reaction and Titration Analysis
A strong acid reacts with a strong base in a titration experiment. Consider the reaction between hyd
Acid-Base Reaction Kinetics: Neutralization Reaction Rate
Acid-base neutralization reactions are fundamental in chemistry. Design an experiment to investigate
Acid-Base Titration Analysis of Hydrochloric Acid
A student aims to determine the concentration of a 0.10 M HCl solution by titrating it with a 0.10 M
Analysis of Solubility Product in a Precipitation Reaction
A student determines the solubility product (Ksp) of a sparingly soluble salt by preparing a saturat
Balancing Half-Reactions in Redox Processes
In the context of redox reactions, several proposed half-reactions for the oxidation of iron (Fe) ar
Decomposition Reaction Analysis
A compound $$AB$$ is known to decompose upon heating, following the reaction: $$AB \rightarrow A + B
Decomposition Reaction and Stability Analysis
Compound AB decomposes when heated according to the reaction: $$AB \rightarrow A + B$$. Experimental
Designing an Experiment for Redox Reaction: Copper Reduction
The reduction of $$Cu^{2+}$$ to metallic copper is a classic redox reaction. Design an experiment to
Effect of Reactant Purity on Precipitate Yield
Impurities in reactants can significantly affect the outcome of a precipitation reaction. Design an
Electrolysis and Faraday’s Laws
During the electrolysis of aqueous copper(II) sulfate using copper electrodes, a constant current of
Error Analysis in Chemical Reaction Yields
To evaluate the efficiency of a chemical reaction, both percent error and percent yield are used. In
FRQ 2: Stoichiometry and Percent Error in Combustion Analysis
In a combustion analysis experiment, a 4.0 g sample of a hydrocarbon is burned, yielding 11.2 g of $
FRQ 19: Designing a Redox Titration Experiment for Unknown Concentration
In a redox titration, $$KMnO_{4}$$ is used to titrate an unknown $$Fe^{2+}$$ solution. The claim is
FRQ 20: Calorimetry and Reaction Enthalpy Analysis
A calorimetry experiment is performed to determine the reaction enthalpy. The expected enthalpy chan
Gravimetric Analysis of Alkali Hydroxide
A 4.33 g sample of an unknown alkali hydroxide is completely dissolved in water. An excess of copper
Gravimetric Analysis of an Unknown Salt
An unknown salt that contains chloride ions is analyzed by precipitating AgCl from a 1.00 g sample u
Gravimetric Analysis: Identification of a Metal Ion via Precipitation
An unknown solution containing a trivalent metal ion \(M^{3+}\) is treated with a base to precipitat
Integration of Reaction Types: From Synthesis to Redox
An industrial process involves two sequential reactions. First, a synthesis reaction produces compou
Interpreting a Reaction Rate vs. Time Graph in a Redox Reaction
A redox reaction is monitored by measuring the concentration of an oxidizing agent over time. The fo
Kinetics of a Decomposition Reaction
The decomposition of hydrogen peroxide ($$H_2O_2$$) is studied in the presence of a catalyst. The re
Multi-Step Stoichiometric Analysis of Consecutive Reactions
A compound undergoes two consecutive reactions. First, it participates in a synthesis reaction to fo
Multi-Step Synthesis and Decomposition Reaction
In a laboratory setting, a compound $$AB$$ is synthesized from elements A and B via the reaction $$A
Polymer Chemistry: Reaction Mechanisms in Polymerization
Polyethylene is produced by the radical polymerization of ethene (C2H4) under high pressure and temp
Quantitative Analysis of a Precipitation Reaction
Barium chloride (BaCl2) reacts with sodium sulfate (Na2SO4) to produce a precipitate of barium sulfa
Quantitative Analysis With Percent Error
Percent error is used to compare experimental yields with theoretical predictions. Design an experim
Redox Reaction and Gas Collection with Pressure Corrections
A student studies the redox reaction between zinc metal and copper(II) sulfate solution by collectin
Redox Titration Data for Determining Concentration of an Unknown
A redox titration is performed using a 0.05 M KMnO₄ solution to determine the concentration of an un
Redox Titration for Concentration Analysis
A redox titration is performed to determine the concentration of an unknown $$Fe^{2+}$$ solution usi
Sequential Reactions: Decomposition and Neutralization
Compound XY decomposes upon heating into element X and diatomic gas Y2. The gas Y2 is then bubbled t
Stoichiometry in Multi-step Reactions
A reactant A undergoes a two-step conversion to product C via an intermediate B. The reactions are:
Synthesis Reaction and Stoichiometric Calculations
An industrial process synthesizes compound AB via the reaction $$A + B \rightarrow AB$$. In a partic
Thermochemistry of Benzene Combustion
Benzene ($$C_6H_6$$) combusts with oxygen to produce carbon dioxide and water. Using the standard en
Analyzing Reaction Mechanisms and Intermediates
Consider the following proposed mechanism for the reaction: Step I (fast): A + A → X Step II (slow)
Arrhenius Equation and Temperature Dependence
A reaction’s rate constant k was determined at various temperatures. The following data was obtained
Arrhenius Plot and Activation Energy Determination
The Arrhenius equation relates the rate constant $$k$$ to temperature $$T$$ and activation energy $$
Arrhenius Plot Interpretation
A series of experiments were conducted to determine the temperature dependence of a reaction rate. T
Comparing Kinetic Orders using Integrated Rate Laws
A chemist investigates the kinetics of a reaction that is suspected to be first order. Three differe
Comparison of Reaction Mechanisms
Two different mechanisms have been proposed for the reaction $$A + 2*B \rightarrow C$$. One mechanis
Derivation of Overall Rate Law for a Pre-Equilibrium Mechanism
Consider the following mechanism: Step I (fast equilibrium): $$A + B \rightleftharpoons C$$ with eq
Determining Arrhenius Constants from Temperature-Dependent Rate Data
A study was conducted to measure the rate constant $$k$$ for a reaction at different temperatures. T
Determining Rate Law from Reaction Mechanisms
A reaction proceeds through multiple steps. The proposed mechanism is as follows: I. A + A ⇌ X (fas
Determining Reaction Order from Initial Rate Data
A chemist investigates the reaction $$A + 2*B + C \rightarrow D$$. The following data were collected
Distinguishing Types of Enzyme Inhibition
A biochemical reaction is studied under different conditions, yielding the following kinetic paramet
Elucidating Reaction Mechanism and Rate-Determining Step
Consider the following elementary reaction mechanism: I. $$A + A \rightarrow X$$ (fast) II. $$X +
Evaluating Alternative Reaction Mechanisms
Two mechanisms have been proposed for the reaction $$\text{A} + \text{B} \rightarrow \text{C}$$: Me
Evaluating Methods to Determine Reaction Kinetics
Two distinct methods for determining reaction kinetics are employed: (1) the method of initial rates
Graphical Analysis of a Second-Order Reaction
For a second-order reaction, the integrated rate law can be expressed as $$\frac{1}{[A]} = k*t + \fr
Graphical Determination of Kinetic Order from Concentration Data
The concentration of reactant A in a reaction is measured over time. Use the data provided to determ
Impact of Stirring on Reaction Rate in Homogeneous vs. Heterogeneous Mixtures
Stirring is often used in chemical reactions to improve mixing. Compare the effects of stirring on r
Influence of Solvent Polarity on Reaction Kinetics
A reaction was carried out in various solvents, and the following data were collected: | Solvent
Influence of Surface Area on Reaction Kinetics in Heterogeneous Mixtures
Heterogeneous reactions often occur at the interface between phases. Answer the following parts rega
Integrated Rate Law Analysis for a Second Order Reaction
A second order reaction was investigated by monitoring the concentration of reactant A over time. Th
Interpreting Reaction Energy Profile Diagrams
A reaction energy profile diagram is provided below. Answer the following questions based on the dia
Kinetic Competition in Parallel Reaction Pathways
In a reaction where A + B can proceed via two parallel pathways to form product C, each pathway has
Kinetic Isotope Effects and Reaction Mechanisms
A recent investigation found that when hydrogen in a reactant is replaced with deuterium, the reacti
Kinetics of a Zero-Order Reaction
The concentration of reactant A is measured over time for a zero-order reaction. The experimental da
Molecular Orientation and Reaction Rates
Collision theory states that in addition to sufficient energy, reactant molecules must have the corr
Photochemical Reaction Kinetics in a Light-Activated Reaction
Certain reactions are initiated by the absorption of light (photochemical reactions).
Reversible Reaction Kinetics: Neglecting the Reverse Reaction
An experiment was designed to study the kinetics of the reaction $$A \rightarrow B$$. The initial ra
Analyzing Environmental Impact of Endothermic Processes
An industrial facility employs an endothermic reaction to capture carbon dioxide from emissions, whi
Analyzing Thermodynamic Data for Reaction Feasibility
A study claims that the measured enthalpy change (ΔH) of a reaction alone can predict its spontaneit
Application of Hess’s Law
A reaction can be carried out in a series of steps. Consider the following steps: Step 1: $$A \righ
Bomb Calorimetry and Reaction Enthalpy Determination
Bomb calorimetry is used to accurately measure the enthalpy change of combustion reactions. Explain
Bond Energy Calculation and Reaction Enthalpy
Consider the reaction: $$2*H_2(g)+O_2(g)\rightarrow 2*H_2O(l)$$. Use the bond energies provided in t
Catalyst Recycling and Energy Savings in Industrial Processes
An industrial chemical process employs a catalyst to reduce the energy consumption of a reaction. Th
Comparison of Heat Flow in Different Materials
A graph compares the temperature change over time for two different substances when the same amount
Determination of Enthalpy of Formation from Experimental Data
Experimental data has been collected to study the formation of compound X from its elements. The for
Effect of Pressure on Enthalpy Changes in Gaseous Reactions
A gaseous reaction is conducted at various pressures to investigate if pressure affects the measured
Energy Analysis in Sublimation Process
A student performs an experiment to measure the energy required for the sublimation of a solid (e.g.
Energy Diagram Analysis of an Exothermic Reaction
An experiment produced an energy diagram for a chemical reaction where the energy level of the react
Environmental Impact of Endothermic Processes
Endothermic reactions absorb energy from their surroundings and can impact the environmental energy
Exploring Enthalpy of Solution
A student dissolves an ionic compound, sodium chloride (NaCl), in water and notices a slight drop in
Frictional Heating and Energy Conversion
In a friction experiment, a block slows down as it slides along a surface and its temperature increa
Interpreting Calorimetry Data from a Metal-Water Reaction
In a calorimetry experiment, a heated metal is immersed in water, and the temperature of the water i
Investigating Heat Transfer in Liquid Mixtures
A researcher performs an experiment by mixing 50.0 g of water at 80.0°C with 50.0 g of water at 20.0
Investigating the Effect of Temperature on Reaction Rates Using Calorimetry Data
A researcher studies an exothermic reaction by performing calorimetry experiments at different tempe
Phase Diagram Analysis and Thermodynamics
A phase diagram for an unknown substance is provided, showing the boundaries between solid, liquid,
Specific Heat Capacity and Heat Transfer in Everyday Materials
In an experiment, the temperature change of water and a metal cup were recorded under identical heat
Thermodynamic Considerations in Industrial Chemical Reactions
A chemical reaction used in manufacturing is moderately endothermic with \(\Delta H = +60 \text{ kJ/
Acid Dissociation Equilibrium Analysis
Consider the dissociation of a weak acid, HA, in water given by: $$HA(aq) \rightleftharpoons H^+(aq)
Advanced Calculation of Kp from Partial Pressure Data in Reforming Reaction
Consider the methane reforming reaction: $$CH_4(g) + H_2O(g) \leftrightharpoons CO(g) + 3*H_2(g)$$.
Calculating Equilibrium Constant from Experimental Data
Outline the process for determining the equilibrium constant (Kₑ𝚚) from experimental concentration
Calculating Q and Predicting Equilibrium Shift
For the reaction $$P \rightleftharpoons Q$$, the equilibrium constant, $$K_{eq}$$, is 20. (a) Write
Combining Reactions and the Equilibrium Constant
Two reversible reactions were studied: Reaction 1: A ⇌ B with $$K_{eq1} = 4.0$$, and Reaction 2: B ⇌
Common Ion Effect on AgCl Solubility
Consider the solubility equilibrium of silver chloride (AgCl): $$AgCl (s) \rightleftharpoons Ag^+ (a
Comparative Analysis of Equilibrium Constants: Kc vs Kp
For the gaseous reaction $$L (g) + M (g) \rightleftharpoons N (g)$$, analyze the relationship betwee
Determining Overall $$Keq$$ from a Reaction Mechanism
A reaction proceeds in two steps: Step 1: $$A \rightleftharpoons B$$ with $$K_1 = 2$$, Step 2: $$B \
Determining Reaction Direction using Q and Keq
A reaction A + 2B ⇌ 3C is studied in the lab. The concentrations at a given moment are provided in t
Dilution and $$K_a$$: Acetic Acid Equilibrium Experiment
A student investigates the ionization of acetic acid by preparing a 0.1 M solution and then diluting
Dilution Effects on Aqueous Equilibrium
A student investigated the equilibrium $$\text{Fe}^{3+} (aq) + \text{SCN}^- (aq) \rightleftharpoons
Dynamic Analysis of Reaction Quotient Over Time
A system undergoing the reaction $$\mathrm{D (aq) + E (aq) \rightleftharpoons F (aq)}$$ is monitored
Dynamic Equilibrium and Reaction Rates
At equilibrium, a reaction exhibits constant concentrations due to balanced forward and reverse reac
Effect of Catalysts on Equilibrium and Reaction Rates
A reaction is catalyzed by an enzyme, which increases the rate at which equilibrium is reached witho
Effect of Dilution on Aqueous Equilibria
Investigate how dilution (adding water) affects the position of equilibrium in aqueous systems.
Equilibrium Analysis in a Multi-Step Reaction
A multi-step reaction consists of two consecutive equilibria: Reaction 1: $$A \rightleftharpoons B$$
Equilibrium in Aquatic Systems: Nitrate Levels Analysis
Following an industrial discharge, the nitrate concentration in a lake was monitored over time. The
Equilibrium in Salt Solubility Calculations
For the dissolution of PbCl₂, represented by: $$PbCl₂(s) \rightleftharpoons Pb²⁺(aq) + 2Cl⁻(aq)$$, t
Equilibrium Shifts in the Haber Process
A chemical plant uses the Haber process for the industrial production of ammonia, where the reaction
Experiment on the Common Ion Effect on Solubility
Design an experiment to investigate how the common ion effect influences the solubility of AgCl.
Impact of Inert Gas Addition on Equilibrium at Constant Volume
A study on the gaseous equilibrium L ⇌ M was performed with the addition of an inert (noble) gas at
Inert Gas Effects on Equilibrium
Consider a gaseous equilibrium system at equilibrium. Describe what happens when an inert gas is add
Interconversion Between Kc and Kp
For the gas-phase equilibrium reaction $$N_2O_4 (g) \leftrightharpoons 2*NO_2 (g)$$, answer the foll
Le Chatelier’s Principle: Concentration Effects on Equilibrium
Design an experiment to study the effect of dilution on the equilibrium of the reaction: $$\mathrm{F
Le Chatelier's Principle: Effect of Concentration Changes
Consider the aqueous equilibrium: $$\mathrm{Fe^{3+} + SCN^{-} \rightleftharpoons FeSCN^{2+}}$$. Answ
Manipulating Equilibrium Through Catalyst Use
Catalysts are frequently used in chemical processes to speed up reactions without altering the posit
Manipulation of Equilibrium Constants via Hess's Law
Given the following reactions with their equilibrium constants: Reaction 1: $$\mathrm{A \rightlefth
Manipulation of Equilibrium Constants via Reaction Reversal and Scaling
For the reaction: $$A+B ⇌ C$$, a researcher knows that the equilibrium constant $$K$$ is 8.0. Answer
Precipitation Reaction Analysis: Mixing of Solutions
A solution of 0.050 M Na$_2$SO$_4$ is mixed in equal volumes with a 0.10 M BaCl$_2$ solution. Barium
Pressure and Volume Effects in Gaseous Equilibria
Analyze the impact of changes in pressure and volume on the equilibrium position of a gaseous reacti
Pressure Effects on Gaseous Equilibrium
Consider the gaseous equilibrium reaction: $$2SO_2(g) + O_2(g) \rightleftharpoons 2SO_3(g)$$ A decre
Quantitative Analysis of the Common Ion Effect on Solubility
A salt, $$AB_{2}$$, dissolves in water according to: $$AB_{2}(s) ⇌ A^{2+}(aq) + 2*B^{-}(aq)$$ with a
Quantitative Equilibrium Calculation in a Weak Acid System
A weak acid, HA, dissociates according to $$HA \leftrightarrow H^{+} + A^{-}$$. In a 1.0 L solution,
Reaction Mechanism and Equilibrium Shift Analysis
A complex chemical reaction proceeds via a two-step mechanism, with the first step achieving a rapid
Reaction Quotient Analysis using Data Table
Analyze the reaction quotient $$Q$$ for an equilibrium system using experimental concentration data.
Reaction Quotient and Equilibrium Adjustment
For a general equilibrium reaction $$\ce{aA + bB <=> cC + dD}$$, the reaction quotient is given by $
Solubility Changes with Temperature: An Experimental Analysis
A series of experiments measured the solubility of a sparingly soluble salt at various temperatures.
Solubility Product and the Common Ion Effect
AgCl is a sparingly soluble salt that dissolves according to the equation: $$AgCl(s) \rightleftharpo
Solubility Product and the Common Ion Effect
The salt $$AB_2$$ dissolves in water according to the reaction: $$AB_2(s) \rightleftharpoons A^{2+}(
Solubility Product Constant (Ksp) Calculation
Define and utilize the solubility product constant (Ksp) to predict the solubility of a sparingly so
Studying the Impact of Pressure Changes on Gas Equilibria
Consider the equilibrium reaction: $$N_2(g) + 3*H_2(g) \rightleftharpoons 2*NH_3(g)$$ Answer the f
Temperature Effects on Equilibrium Constants
Examine how temperature variations affect the equilibrium constants for both exothermic and endother
Common Ion Effect on Solubility of Mg(OH)₂
This question examines the impact of the common ion effect on the solubility of magnesium hydroxide.
Common Ion Effect on the Solubility of Mg(OH)2
In a saturated solution of Mg(OH)2, the following equilibrium is established: $$Mg(OH)_2 (s) \rightl
Computational Modeling of Acid-Base Equilibria
A researcher develops a computer simulation to model complex acid-base equilibria in aqueous solutio
Designing a Titration Laboratory Experiment for a Weak Acid
A researcher is tasked with designing a titration experiment to determine the concentration of a wea
Designing an Experiment to Investigate pH Dependence on Hydroxide Salt Solubility
This FRQ requires you to design an experiment to investigate how the pH of a solution affects the so
Indicator Color Change and pH Transition Range
Indicators are weak acids whose color changes with pH. Analyze their role in acid–base titrations.
Indicator Selection and Color Change Analysis in Titrations
In a series of titration experiments involving a weak acid and a strong base, different pH indicator
Industrial pH Adjustment in Wastewater Treatment
A wastewater treatment plant is tasked with neutralizing acidic effluent before discharge. The efflu
Neutralization Reaction Analysis
Compare the reaction mechanisms and outcomes for the following neutralization reactions: (1) Strong
Polyprotic Acid Equilibrium
Phosphoric acid (H₃PO₄) is a polyprotic acid with dissociation constants given as: $$K_{a1} = 7.1
Polyprotic Acid Titration and Stepwise Dissociation
Phosphoric acid (H3PO4) is a polyprotic acid that dissociates in three steps with the dissociation c
Predicting pH Changes on Addition of a Base to a Polyprotic Acid Solution
This FRQ focuses on predicting the pH change when a strong base is added incrementally to a polyprot
Relationship Between $$K_a$$, $$K_b$$, and $$K_w$$
For a conjugate acid-base pair, the relationship $$K_a \times K_b = K_w$$ holds true. Given a weak a
Role of Weak Bases in Buffer Systems
Weak bases are often used in buffer systems to help maintain pH stability. Address the following:
Solubility and pH Interdependence for Basic Salts
Magnesium hydroxide, Mg(OH)₂, has a Ksp of $$5.61 \times 10^{-12}$$. (a) Calculate the molar solub
Stoichiometric Analysis of Neutralization
A solution containing 0.04 mol of HCl is mixed with 0.03 mol of NaOH, and the final volume of the so
Titration Curve Simulation and Data Analysis
Simulated titration data for a weak acid titrated with a strong base is provided. Analyze the titrat
Understanding pH Beyond the Conventional Range
A study asserts that under extreme experimental conditions, the pH of a solution can fall below 0 or
Analyzing a Voltaic Cell's Performance Over Time
A report claims that the voltage output of a voltaic cell remains constant over several hours of ope
Assessing Electroplating Efficiency with Miscalculated Electron Moles
In an electroplating experiment designed to deposit a thin metal coating on a substrate, a researche
Calorimetry Misapplication in Thermodynamic Measurements
In an attempt to determine both the enthalpy (\(\Delta H^\circ\)) and entropy (\(\Delta S^\circ\)) c
Comparing Spontaneity in Redox vs. Non-redox Reactions
A complex chemical process involves coupling a spontaneous redox reaction with a non-spontaneous rea
Designing a Galvanic Cell
A galvanic cell is constructed using a copper electrode immersed in 1 M CuSO₄ and a zinc electrode i
Designing an Experiment to Measure Gibbs Free Energy Changes
A chemist plans to determine the standard Gibbs free energy change (ΔG°) for a reaction by measuring
Effect of Phase Change on Entropy
Consider the following two reactions: (1) $$N_2(g) + 3H_2(g) -> 2NH_3(g)$$ (2) $$SO_3(g) + H_2O(g) -
Electrochemical Cells: Galvanic Cell Analysis
A researcher builds a galvanic cell by combining two half-reactions and measures a standard cell pot
Electroplating and Faraday's Law Application
An electrolytic cell is used for electroplating copper. A current of $$2.0\,A$$ is applied for $$600
Entropy Considerations in a Reaction with Phase Change
For the reaction $$CaCO_3(s) \rightarrow CaO(s) + CO_2(g)$$, analyze the entropy change.
Evaluating Electrolysis Efficiency
An electroplating experiment operating at a current of 5.00 A for 2 hours resulted in 10.0 g of meta
Evaluating Reaction Conditions for Electrolysis
An electrolytic cell is used to decompose water with a constant current of $$1.5\,A$$ applied for $$
FRQ 8: Entropy Change Due to Mole Change and Phase Transition
Consider the reaction involving the splitting of water: $$2\,H_2O(l) \rightarrow 2\,H_2(g) + O_2(g)
Gibbs Free Energy and Equilibrium Constant Relationship
For the reaction $$N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g)$$ the equilibrium constant is given
Gibbs Free Energy Calculation and Reaction Analysis
A media headline claims that a certain reaction is thermodynamically favored at room temperature bec
Graph Analysis: Determining Enthalpy Change
A graph depicting a linear relationship between \(\ln K\) and \(1/T\) has been obtained from experim
Interpretation of a Galvanic Cell Diagram
Examine the provided diagram of a simple galvanic cell.
Investigating the Effect of Pressure on Reaction Entropy
Design an experiment to measure the impact of pressure on the entropy change of a gas-phase reaction
Modeling Reaction Favorability Using Thermodynamic Data
Using a provided table of ΔH° and ΔS° values, determine the reaction favorability for several reacti
Predicting Reaction Spontaneity using Temperature and Entropy
Consider a reaction with \(\Delta H° = +30 \text{ kJ/mol}\) and \(\Delta S° = +120 \text{ J/mol·K}\)
Pressure Effects on Gibbs Free Energy
In gas-phase reactions, pressure changes can influence the Gibbs free energy. Consider a reaction th
Redox Cell Salt Bridge Function Error
In an effort to measure the cell potential of a galvanic cell, a researcher set up a redox cell usin
Reduction Potentials and Reaction Feasibility
A researcher analyzes two half-reactions with standard reduction potentials provided below. Using th
Temperature Dependence of Gibbs Free Energy
An experiment is conducted to study the effect of temperature on the spontaneity of a reaction that
Temperature Effects on Electrolysis and Electroplating
In an experiment involving an electrolytic cell used for electroplating, the effects of temperature
Thermodynamic Favorability: Interplay of $$\Delta H$$ and $$\Delta S$$
Examine the following reaction data and use it to analyze thermodynamic favorability from the relati
Unit Conversion in Gibbs Free Energy Calculations
A reaction has \(\Delta H° = -100 \text{ kJ/mol}\) and \(\Delta S° = -210 \text{ J/mol·K}\) at 298 K
Trusted by millions
Everyone is relying on Knowt, and we never let them down.



Need to review before working on AP Chemistry FRQs?
We have over 5 million resources across various exams, and subjects to refer to at any point.
Explore Top AP Chemistry Flashcards
Explore Top AP Chemistry Notes
Browse top AP materials
We’ve found the best flashcards & notes on Knowt.
Explore top AP flashcards
Explore top AP notes
Tips from Former AP Students
FAQ
We thought you might have some questions...
- Of course, make sure to run through College Board's past FRQ questions!
- Once you’re done with those go through all the questions in the AP ChemistryFree Response Room. You can answer the question and have it grade you against the rubric so you know exactly where to improve.
- Reddit it also a great place to find AP free response questions that other students may have access to.