Kinetics
Kinetics
- How quickly something happens (PharmD and the rate of a release of a drug from a capsule)
- Rate is how quickly something is performed.
In a chemical reaction, as it proceeds, initially reactants are turning into products
The graph of these initial changes are:
- In words, the rates can be expressed as:
- the formation of products/time
- or disappearance of reactants/time (-ve sign)
The change in reaction rate per time is most commonly expressed as:
- Change in conc/unit time in mole dm^-3s^-1
- This can be taken from a tangent to a curve off of a graph at several points with reference to the time.
Sometimes the disappearance of reactants per time are at different rates which can be seen in graph form and is indicated by A and B and their coefficients in a chemical equation:
- 2A + B → C
The shape of curve indicates that the rate of a reaction is not constant during the reaction but is fastest at the start and slows down as the reaction proceeds.
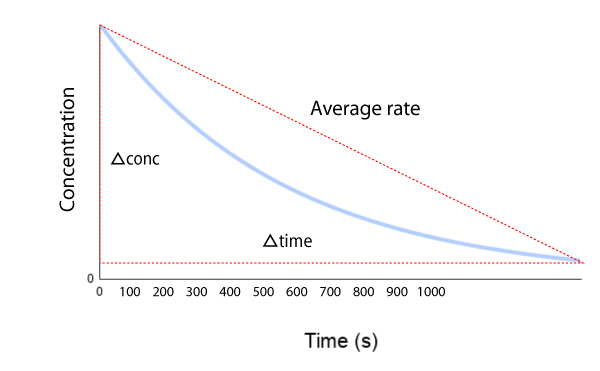
The change in rate has to do with the effects of the change in concentration.
We measure initial rates by drawing a tangent to the graph at t=0
As a reaction proceeds, the slope of a concentration time graph decreases.
Ways to measure rates of reactions
- Change in volume
- Change in mass
- Change in light transmission (spectroscopy)
- Change in concentration using titration to stop it (quenching)
- Change in conductivity (graphed)
- Change in color (clock reactions)
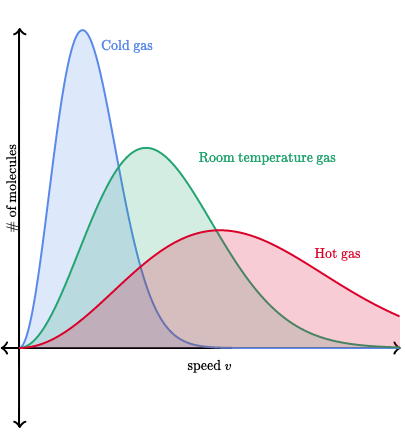
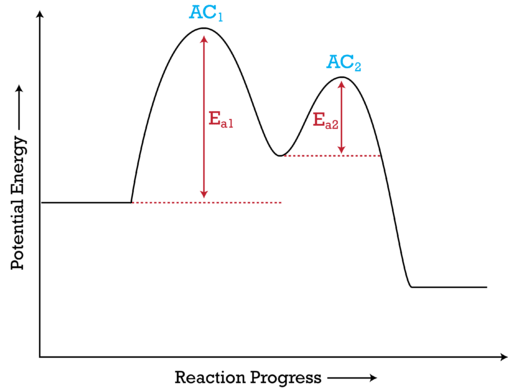
Example
| Experiment | [NO(g)] mol dm^-3 | [CO(g)] mol dm^-3 | [O2(g)] mol dm^-3 | Initial rate / mol dm^-3 s^-1 |
|---|---|---|---|---|
| 1 | 1.00x10^-3 | 1.00x10^-3 | 1.00x10^-1 | 4.40x10^-4 |
| 2 | 2.00x10^-3 | 1.00x10^-3 | 1.00x10^-1 | 1.76x10^-3 |
| 3 | 2.00x10^-3 | 2.00x10^-3 | 1.00x10^-1 | 1.76x10^-3 |
| 4 | 4.00x10^-3 | 1.00x10^-3 | 2.00x10^-1 | 7.04x10^-3 |
Rate = k[NO]^2[H2]
| Experiment | Initial [C4H9Br] / mole dm^-3 | Initial [OH-] / mol dm^-3 | Initial rate of reaction / mol dm^-3 min^-1 |
|---|---|---|---|
| 1 | 0.010 | 0.010 | 2.0x10^-3 |
| 2 | 0.020 | 0.010 | 4.0x10^-3 |
| 3 | 0.020 | 0.020 | 4.0x10^-3 |
Determining the value of rate constant “k”
Rate = k[C4H9Br]
.002M/min = k(.01M)
.002/.01 = k
K = 0.200/minute
H2 + NO → X fast
X + NO → Y + H2O slow
Y + H2 → N2 + H2OZ fast
Rate = k[NO]^2[H2]