Unit 9: Applications of Thermodynamics
Entropy
- Entropy, S, is the amount of disorder or chaos in a system. More disorder, greater S value.
- Standard entropy is S° and measured at 25 celsius
- Standard entropy change ∆S° is measure at the end of a reaction
- ∆S° = (sum of ∆S° products) - (sum of ∆S° reactants)
- If a reaction goes from less moles to more moles (such as 2 moles on the reactant side to 3 moles on the product side) there is more disorder and a positive ∆S
- If a reaction goes from a gas to liquid, liquid to solid, or gas to solid, the reaction has a negative ∆S
- If bonds are broken and phase change becomes more disordered, the ∆S is positive
Gibbs Free Energy
- ∆G is Gibbs Free Energy which determines if a process is thermodynamically favored or unfavored, also known as spontaneous or nonspontaneous
Free Energy Change
- Standard free energy change, ∆G°, is calculated the same as ∆S°
- ∆G° = (sum of ∆G° products) - (sum of ∆G° reactants)
- For a reaction,
- If ∆G is negative, it is TFP (thermodynamically favored process)
- If ∆G is positive, it is not TFP
- If ∆G is 0, it is at equilibrium
∆G, ∆H, and ∆S
- TFP must result in decreasing enthalpy, increasing entropy, or both
- ∆G° = ∆H° - T∆S°
- T = temperature in Kelvin
- ∆S° is usually given in j/mol*K and must be converted to kj/mol*K
- Gibbs Free Energy is usually kj/mol*K
| ∆H | ∆S | T | ∆G | Favorability |
|---|
| - | + | LowHigh | -- | Always TFP |
| + | - | LowHigh | ++ | Never TFP |
| + | + | Low High | +- | Not TFPTFP |
| - | - | Low High | -+ | TFPNot TFP |
Standard Free Energy Change and the Equilibrium Constant
- Gibbs free energy can be calculated if equilibrium constant is known
- ∆G° = -RT(ln K)
- R = gas constant (8.31 j/mol*k)
- T = kelvin temperature
- K = equilibrium constant
- If ∆G° is negative, K is greater than 1, the products are favored at equilibrium
- If ∆G° is positive, K must be less than 1, the reactants are favored at equilibrium
Reduction Potentials
- Every half reaction has electric potential. Potentials are given as reduction half-reactions. If the reaction is reversed, flip the sign to get the oxidation potential
Galvanic Cells
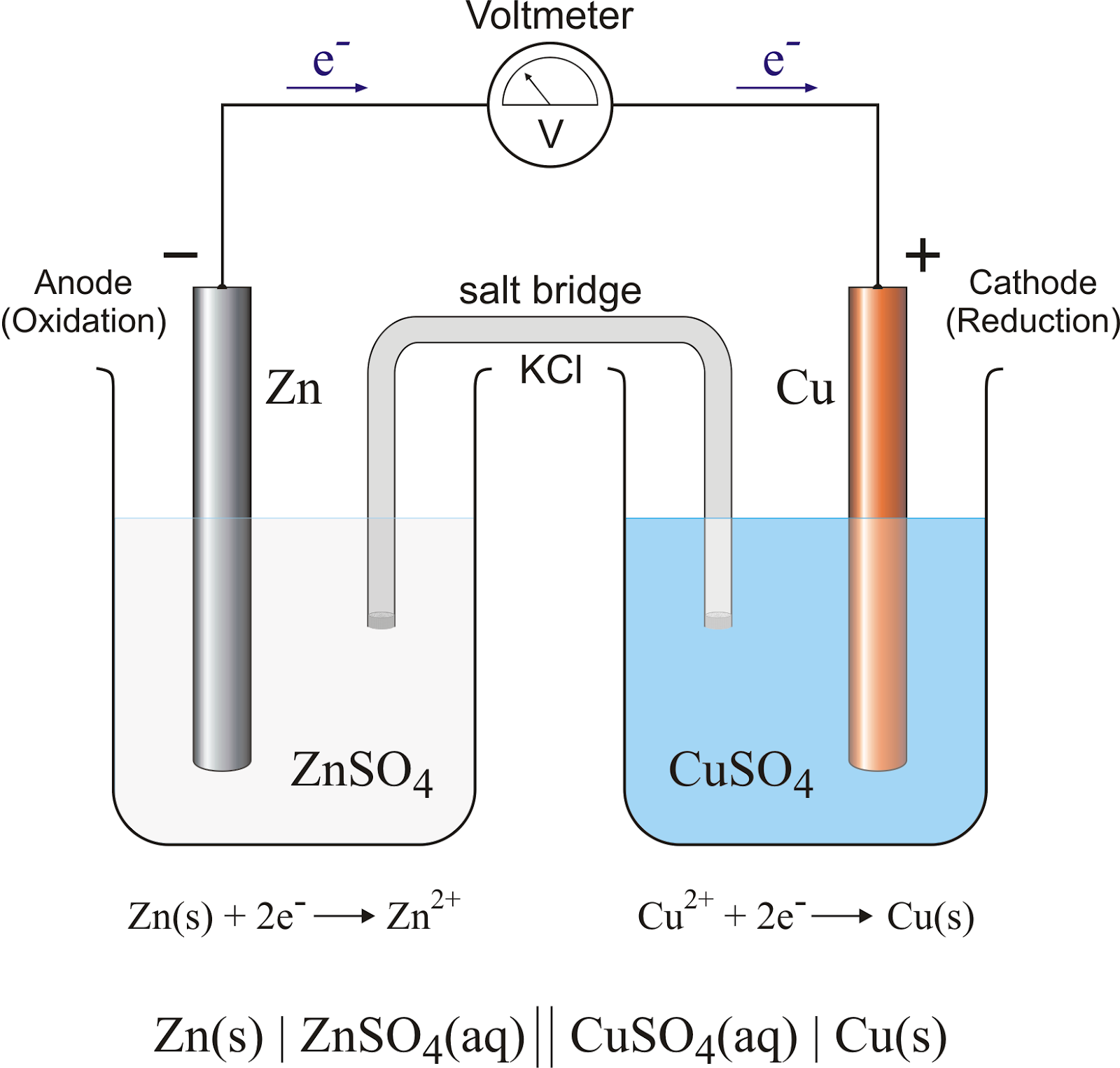
- Galvanic cells (voltaic cell) use favored redox reactions to generate current
- Two half-reactions take place in separate chambers and the electrons from the oxidation pass to the reduction reaction which creates the current
- Current is defined as the flow of electrons from one place to another
- Oxidation takes place at the anode electrode and reduction takes place at the cathode electrode
- The salt bridge keeps electrical neutrality. Without the salt bridge the voltage would be zero. The potassium ion flows to the cathode and the chlorine flows to the anode.
- The cell voltage is equal to the total redox reaction voltage.
Non-Standard Conditions
- Reduction potentials are give at standard conditions, 25 celsius, 1 atm, and 1 M
- Voltaic cells are very favored with equilibrium constant greater than 1. If the Q = K however, the voltage would drop to ero.
- If the reaction quotient increased it would become close to the equilibrium constant and the voltage would decrease.
Electrolytic Cells
- Electrolytic cells use outside voltage sources to power unfavored redox reactions and mainly occur in aqueous solutions.
- The sign of total cell potential is always negative
Electroplating
- Electrolytic cells are used for electroplating.
- I = (q/t)
- I = Current (amperes, A)
- q = charge (coulombs, C)
- t = time (second, s)
- Moles of electrons = (coulombs/ 96,500 coulombs per mol)
Voltage and Favorability
- Redox is favored if the potetial has a positive value. reaction potential can be calculate gibb’s free energy
- ∆G° = -nFE°
- n = number of moles of electrons exchanged in the reaction
- F = Faraday’s constant. 96,500 coulombs/mol
- E° = standard reaction potential (V)
- If E° is positive, ∆G° is negative and is TFP
- If E° is negative, ∆G° is positive and not TFP