Unit 1: Atomic Structure and Properties
The Periodic Table
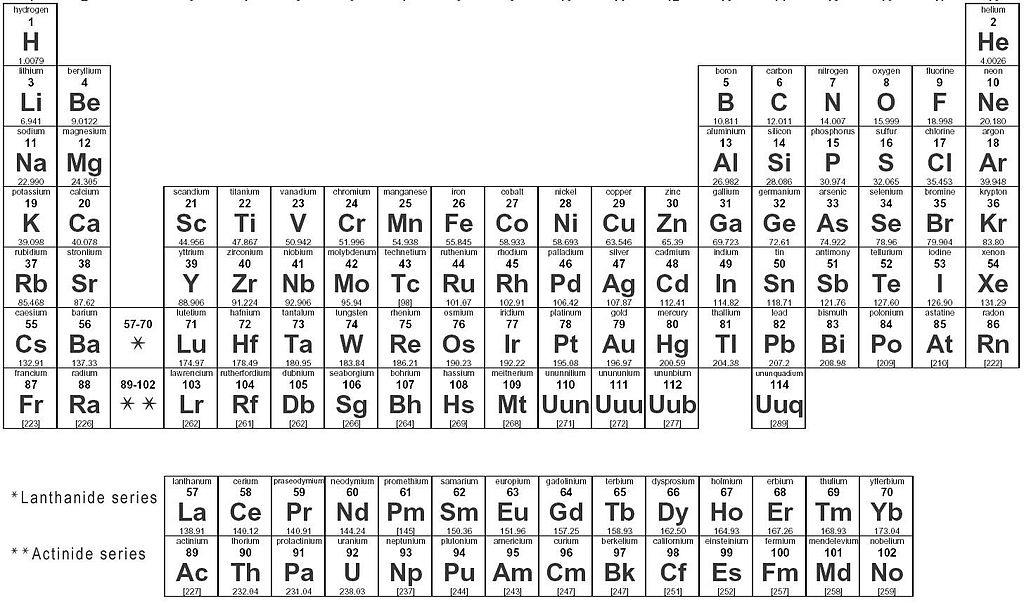
The periodic table gives you very basic but essential information about each element, take carbon for example:
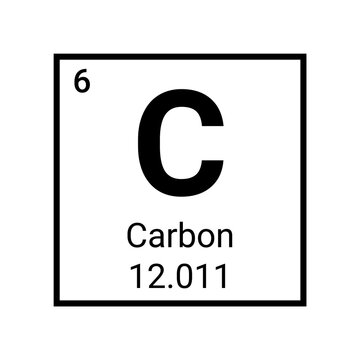
Pictured above is the element carbon as displayed on the periodic table
- The symbol (C) tells you the name: carbon. C and carbon can be used interchangeably
- The atomic number (6) tells you how many protons and neutrons in the element in addition to the amount of electrons surrounding the element when it is neutrally charged
- The molar mass (12.011) tells you two things:
- The average atomic mass of a single atom of carbon, measured in atomic mass units (amus)
- The average mass of a mole of carbon atoms, measured in grams. 12.011 is how many grams of carbon atoms there are in a mole.
Horizontal rows of the periodic table are called periods
Vertical columns are called groups
- Groups can be numbered in Roman numerals or simply as 1-18, but some are also named
- Group IA/1-- Alkali Metals
- Group IIA/2 -- Alkaline Earth Metals
- Group B/3-12 -- Transition Metals
- Group VIIA/17 -- Halogens
- Group VIIIA/18 -- Noble Gases
There is also two rows beneath the periodic table, the lanthanides and actinides, the rare Earth elements, or inner transition metals.
The identity of an atom is determined by the amount of protons in the nucleus, which also contains neutrons. The mass number of atom is the sum of its neutrons and protons (valued at 1 each). Electrons have significantly less mass than protons and neutrons and do not contribute to the mass.
Isotopes are atoms of an element with different numbers of neutrons, but same amount of protons. For example, carbon-12 has 6 protons and 6 neutrons, but carbon-14 has 6 protons and 8 neutrons. The molar mass listed on the periodic table is determined by the average of the mass numbers of all known isotopes of an element weighted by their percent abundance.
The mass of various isotopes of an element can be determined by a technique called mass spectrometry. Below is a mass spectrum for selenium
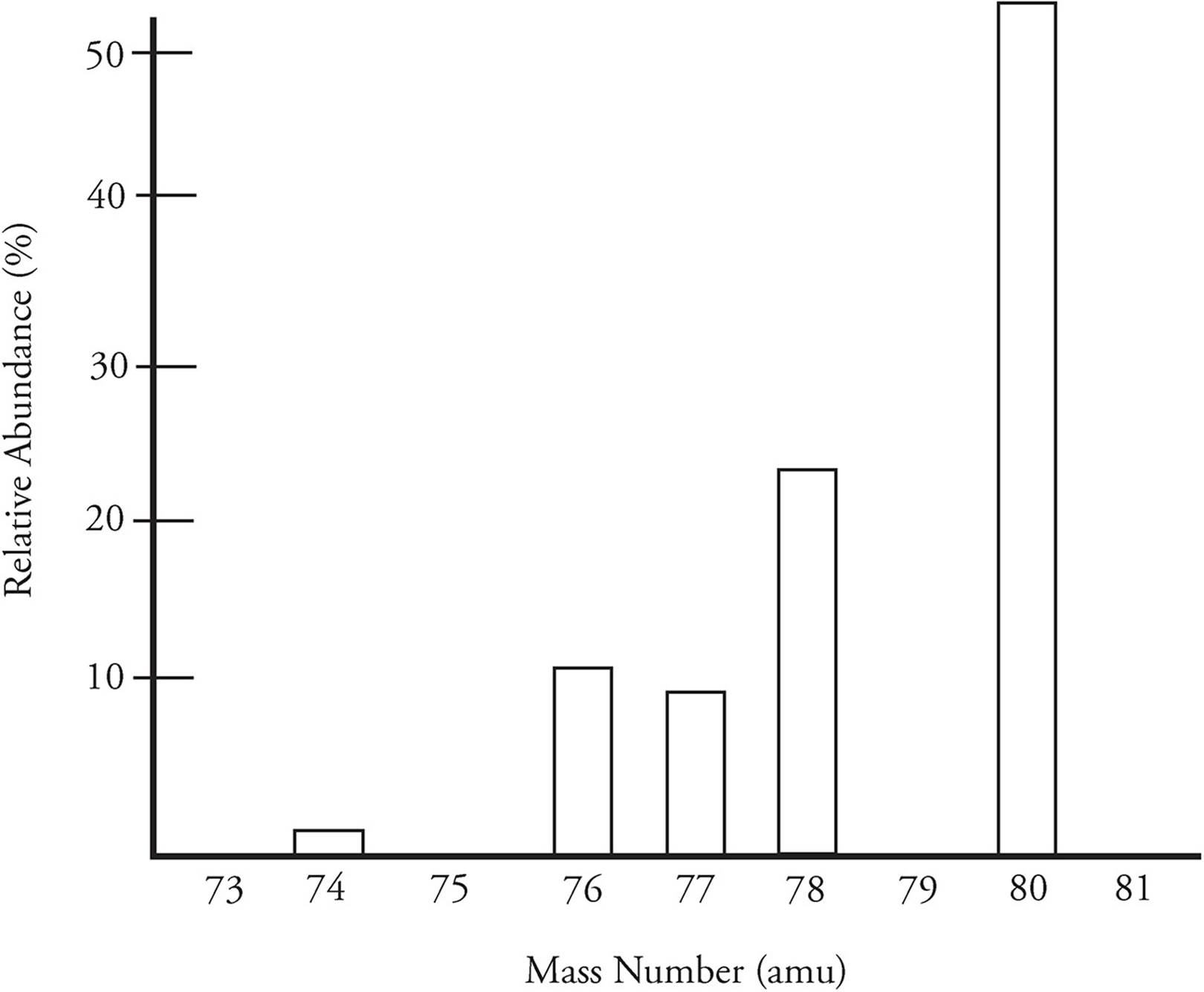
The most abundant isotope of selenium has a mass of 80, but there are four other naturally occurring isotopes. The five isotopes are combined to created a weighted average, which is then the average atomic mass of selenium.
- The molar mass of an element will give you a general idea of the most common isotope. Carbon’s molar mass is 12.01, and 99 percent of all carbon in existence is carbon-12.
Moles
- The quantity ‘mole’ connects all of the different quantities in chemical equations. Coefficients in chemical equations tells you how many moles there are of that substance.
Moles and Molecules
- Avogadro’s Number is the number of atoms in a single mole of any given element. Similar to how a dozen is always 12, Avogadro’s Number is always 6.022x10^23.
- 1 mole = 6.022x10^23 particles
- Moles = particles/(6.022x10^23)
Moles and Grams
- You can convert from moles to grams and vice versa by looking at an elements atomic mass. Atomic mass is given in atomic mass units (Amu), which also signifies grams.
- Example: one carbon atom has a mass of 12.011 amu, therefore, one mol of Carbon atoms has a mass of 12.011 grams.
- Moles = grams/ molar mass
Moles and Gases
- When you know the properties of a gas, you can use the ideal gas law equation to solve for moles.
- PV=nRT where P = pressure in atm, V = volume (liters), n = mol, R = .0821 (ideal gas constant), T = temperature (kelvin).
- However, many gas equations take place at STP, or standard temperature and pressure, where P =1 atm, T=273 Kelvin. at STP converting is easier because one mol ALWAYS occupies 22.4 liters.
- Moles = liters/(22.4 L/ mol)
Molarity
- Molarity (M) expresses the concentration of a solution in terms of volume. Molarity is used in equilibrium calculations, acids and bases, electrochemistry, and more. When an element is shown in brackets, it's being referred to in terms of Molarity.
- [Na+] means "the molar concentration of sodium ions".
Percent Composition
Percent composition is the percent by mass of each element in a compound. To do this, you must divide the mass of each element or component in the compound by the total mass of the compound.
Example: calculate the percent composition each element in calcium nitrate Ca(NO3)2.
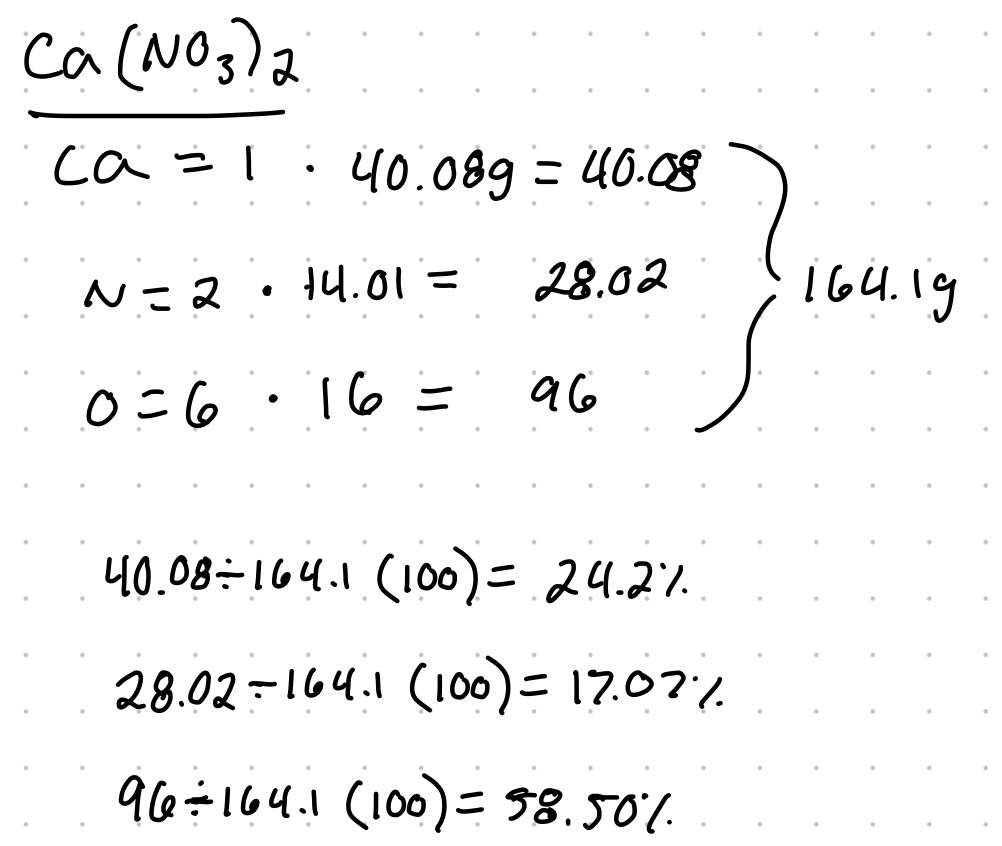
You can add up the percentages to check your work.
Empirical and Molecular Formulas
When given masses or percent mass, you need to be able to convert that to empirical and/or molecular formulas. The empirical formula is the simplest ratio of one element in a compound to another (CH2O). The molecular formula represents the actual formula for the substrate (C6H12O6).
Example: A compound is found to contain 56.5% carbon, 7.11% hydrogen, and 36.4% phosphorus. Part A: determine the empirical formula for the compound.

Part b: if the molar mass is 170.14 g/mol, what's the molecular formula?
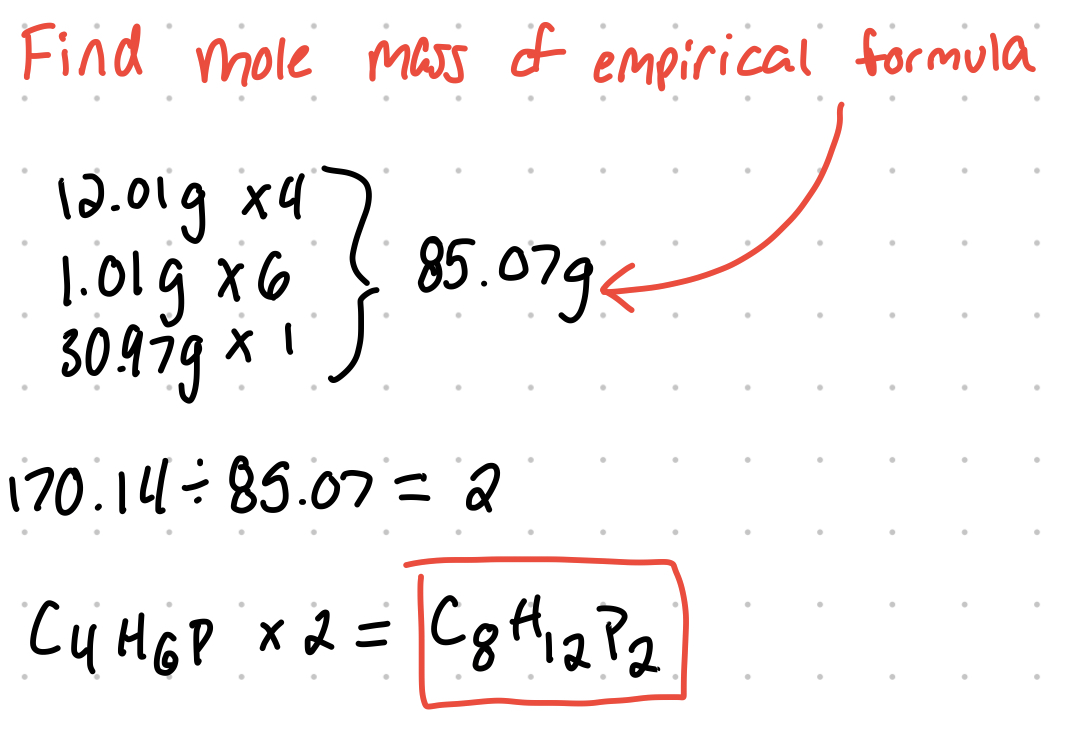
Electron Configurations and the Periodic Table
- The positively charged nucleus is constantly attracting the negatively charged electrons. The farther an electron is from the nucleus, the more the potential energy is.
- The energy of electrons is quantized, which means electrons can only exist at certain energy levels, separated by specific intervals.
Coulomb's Law
The electrostatic force is the attraction between opposite charges and the strength can vary depending on now for the charges are from each other. To find the strength, you can use Coulomb's law
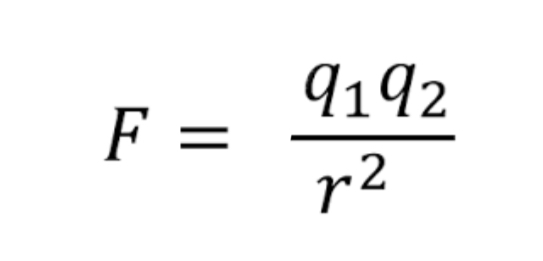
The image of shows the formula for coulomb's law, where F= electrostatic force between nucleus and electron, q1 = magnitude of the positive charge, q2 = magnitude of the negative charge, and r= distance between charges.
Essentially, Coulomb's law states the closer an electron is to the nucleus, the stronger the attraction and the less potential energy there is. in order to remove an electron from an atom, you must add enough energy to overcome the electrostatic force (the binding energy which is always a positive value).
The Bohr Model
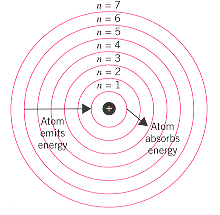
- Each energy level correlates to a row on the periodic table. The closer an energy level is to the nucleus, the less electrons that can be in that level. The Bohr model serves the purpose of understanding atomic structure rather than structural accuracy.
- When electrons absorb electromagnetic radiation, a form of energy, the electrons can jump to higher energy levels. When electrons drop in energy levels, the emit electromagnetic radiation.
Photoelectron Spectroscopy
- To eject an electron from an atom, the ionization energy must be reached in order overcome the binding energies of other electrons in the atom. When examining the spectrum of a single atom, or a small amount atoms, it will be measured in electronvolts (EV), where 1EV= 1.6x10^-19 joules. If measuring moles of atoms, use kJ/mol or MJ/mol.
- When the incoming radiation energy overcomes the binding energy and ejects the electron and there's still radiation left over, the radiation will be converted into kinetic energy for the ejected electron.
- Incoming radiation energy= binding energy + kinetic energy.
- More kinetic energy means faster an ejected electron is moving. The farther an electron is from the nucleus, the less energy it takes to eject. How far an electron is from the nucleus can be determined by looking at the speed of the electron.
Spectra
A photoelectron spectrum (PES) is a graph of the ionization energies for all electrons when ejected from the nucleus.
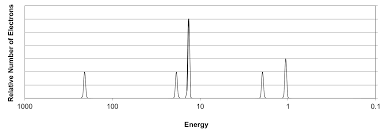
The y-axis, relative number of electrons, is the amount of electrons ejected from a specific energy level.The x-axis shows the binding energy of those electrons which decreases left to right.
Each section of the peaks represents a different energy level and each gap signifies a change in energy level. Because there are multiple peaks in each section, this shows subshells that have different distances. A subshell is the shape of the space an electron can be found in.
The first subshell is the s-subshell that can hold 2 electrons. Second is the p-subshell that can hold six electrons, as shown in the graph by being three times as tall. The height can help determine the amount of electrons in a shell.
In the area for the 3rd energy level, the p-subshell isn't as tall as the other, indicating a partially filled energy level.
Electron Configuration
The periodic table is divided into four subshells, with the size of the atom determining how many subshells it has. Subshells are known as s, p, d (10 max electrons), and f (14 electrons).
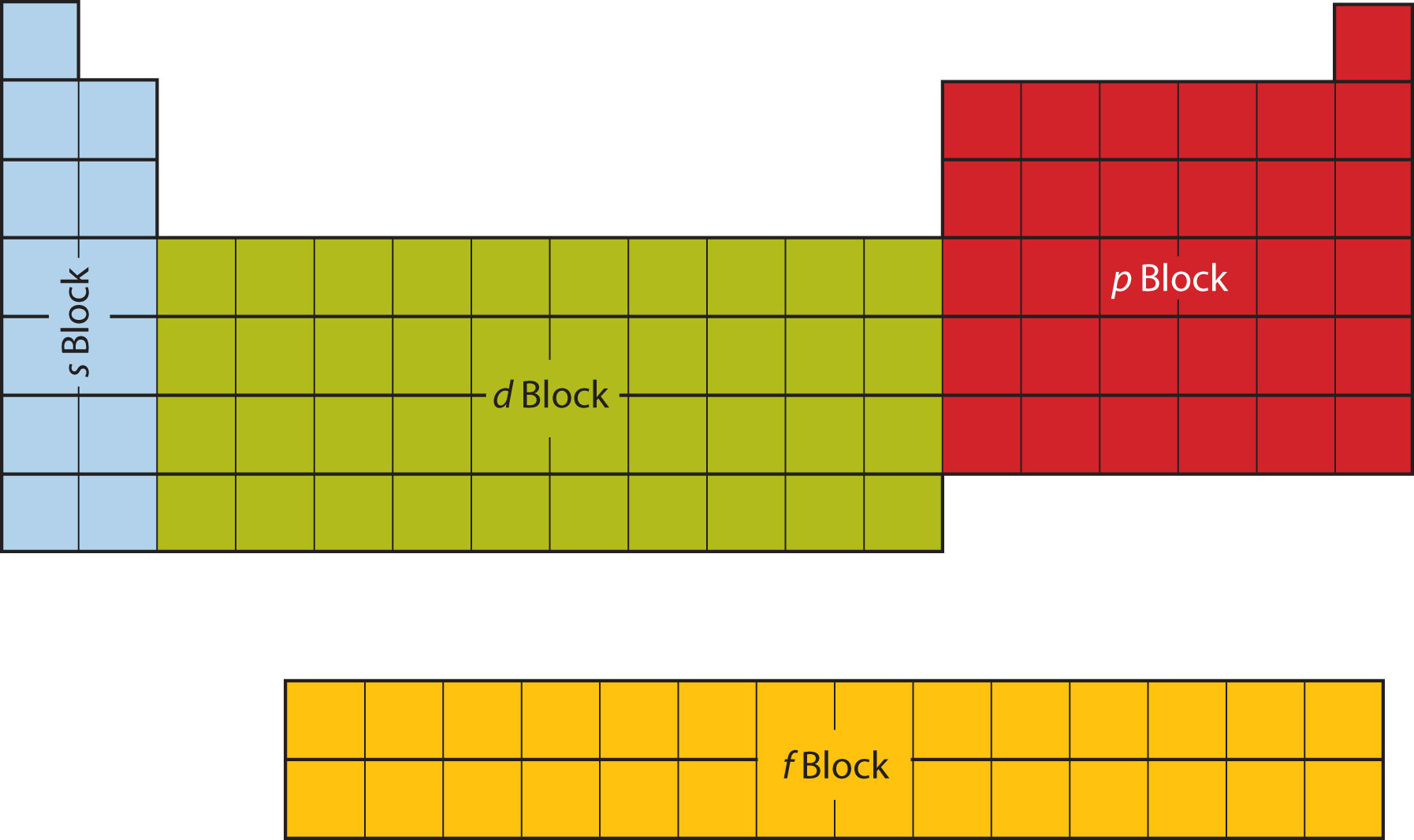
Each subshell is as long as there are electrons in it (s block can have 2 electrons and is two elements long). This method of organization is called electron configuration and can also be used to describe elements.
Configuration Rules
The Aufbau Principle
- Aufbau principle says electrons must fill up orbitals, subshells, and shells in order of increasing energy.
The Pauli Exclusion Principle
- The Pauli exclusion principle states that when two electrons fill up an orbital, they must spin in opposite directions: clockwise and counterclockwise.
Hund's Rule
According to Hund's Rule, when electrons are filling the orbitals of the subshell, they will only have two in one orbital when it is not possible to have each electron in its own orbital.
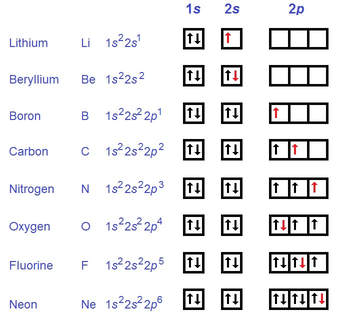
Notice in the above picture howthe arrows are pointing in different directions, signifying different spin, and the arrows will only pair up when there's no other free orbitals (nitrogen and oxygen).
Predicting Ionic Charges
The most stable configuration of an element involves having a completely full outer shell. For any element in the s or p block, that means gaining a total of eight electrons in the outer shell (2 in the s, 6 in the p), known as valence electrons.
When an element gains or loses electrons to become stable they are called ions. when an element is close to a full shell, such as the halogens, they will gain electrons and be negatively charged, these are referred to as anions.
- Halogens as ions will be referred to as Cl^- or F^-1
- Elements in the oxygen group need 2 electrons for stability, so as ions they have a charge -2 (O^-2)
When elements such as the Alkali Metals want to reach stable configuration, they will lose electrons because it takes less electrons than trying to gain. These are called cations when they are positively charged.
Alkali metals have an ionization charge of +1, and alkaline earth metals have an ionization charge of +2, and so on.

Transition metals are cations, but can have multiple different charges which depends on which compound they are in.
- For example, in CuBr2, bromine has a charge of -1 but because there are two bromide ions, that makes their charge -2. in order to have a stable compound, the charge must be zero, which causes the copper charge to be +2. This would be called copper (ii) bromide.
- CuBr would be copper (i) bromide.
Only zinc (+2) and silver (+1) are the only transition metals with one charge possibility.
Periodic Trends
There are three basic rules to understanding trends in the periodic table that help define element behaviors:
- Electrons are attracted to the protons in the nucleus
- The attraction can increase by having a smaller distance between the electron and proton and by having more protons in the nucleus
- Electron shielding is when electrons in the inner shells repel electrons in outer shells because they have the same charge, thus decreasing the attraction between the outer electrons and protons
- Completed shells are stable and elements will try to lose/gain electrons to complete shells, if possible.
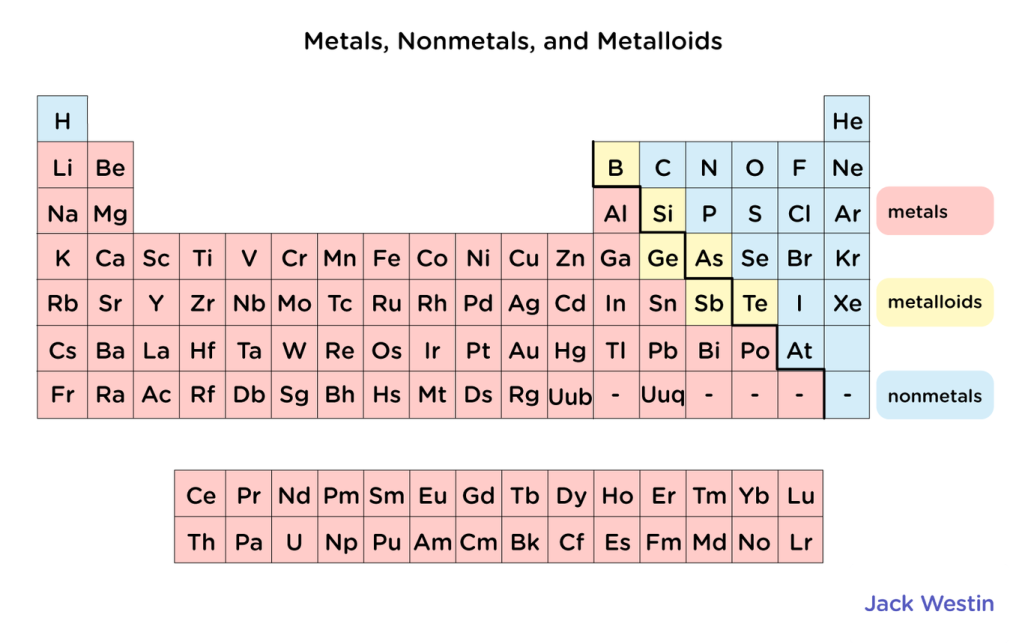
Elements are defined into three categories: metals, metalloid, and nonmetals.
- Metals are on the left side of the periodic table, they give up electrons when forming bonds
- As the metals go more to the right, the less metallic they behave
- Lithium behaves more metallic than zinc.
- Nonmetals are on the right side and gain electrons in bonds
- Metalloids, which have a metallic and nonmetallic character, separate metals and nonmetals.
Atomic Radius
- The approximate distance between the nucleus of the atom to the valence electrons
- Moving from left to right, atomic radius decreases. For example, Lithium has a smaller atomic radius than Fluorine. As the atomic number increases, so does the amount of protons and electrons. Because there are more protons in the nucleus and the same amount of electron shells--despite there being more electrons--there is less of an electron shielding effect.
- Moving down a group, such as from Lithium to Cesium, the atomic radius increases because even though there’s a stronger positive charge, there are more electron shells that increases the shielding effect and pushes electrons farther away.
- Cations are smaller than atoms because electrons are being taken away, electron-electron repulsions are reduced, and there is a stronger positive than negative charge.
- Anions are larger than atoms because the atom has an overall negative charge, increasing the amount of electron-electron repulsions.
Ionization Energy
- To remove electrons from an atom, it requires energy, called the first ionization energy, and creates a cation. To remove another electron, that’s the second ionization energy, and etc.
- Left to right, the ionization energy increases because electrons are closer to the nucleus (protons) and it takes more energy to pull them away from the positive charge.
- Ionization energy decreases when moving down a group because there are more electron shells that cause electron shielding and repulsion, causing the electrons to be not as attracted to the nucleus and easily removed.
- The second ionization energy is greater than the first because the proton to electron ratio has a stronger favor for protons, this causes the electrons to have a stronger attraction than previous and a higher energy is needed to remove a second electron.
- Once a shell is empty, the ionization energy greatly increases because it is closer to the nucleus.
Electronegativity
- Electronegativity is how much an element’s nucleus attracts electrons. Two factors contribute to electronegativity: element size (the smaller the more electronegative) and how close an element is to completing a shell.
- Left to right, electronegativity increases
- Up to down, electronegativity decreases.
- Fluorine is the most electronegative element because it is the smallest element that requires only one electron to complete the shell.