Unit 2: Molecular and Ionic Compound Structure and Properties
Bonds Overview
- Atoms engage in bonding to share electrons and achieve a stable, lower-energy state. This bonding can either be ionic where an atom gives the electron away, or it can be covalent where they share the electron.
Ionic Bonds
Ionic bonds are between a nonmetal and metal held together in a lattice structure by electrostatic attraction. To bond, the cation gives up the electron(s) entirely to the anion.
- The elements are held together by electrostatic force because the positively charged cation is attracted to the negatively charged anion.
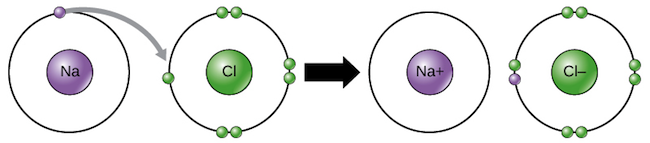
Any substance held together by ionic bonds will be strong, usually solid at room temp, and very high melting and boiling point.
- Melting point for ionic substances can vary depending on the charge. Per Coulomb's Law, a greater charge leads to greater bond energy (MgO with a charge of +2/-2 has a stronger bond than NaCl with a charge of +1/-1).
- if the charges are the same, then the size is considered. The smaller the ions, the greater the bond energy.
Ionic solids in the lattice structure are poor conductors because the electron is stuck around the anion. Liquids are good conductors because the electrons are free to move despite being locked around one atom. Salts are held together by ionic bonds.
Metallic Bonds
Rather than the electron being stuck around one atom, like in ionic bonds, metallic bonds have a "sea of electrons”. The valence electrons freely move about the bond, making them good conductors, and malleable and ductile.
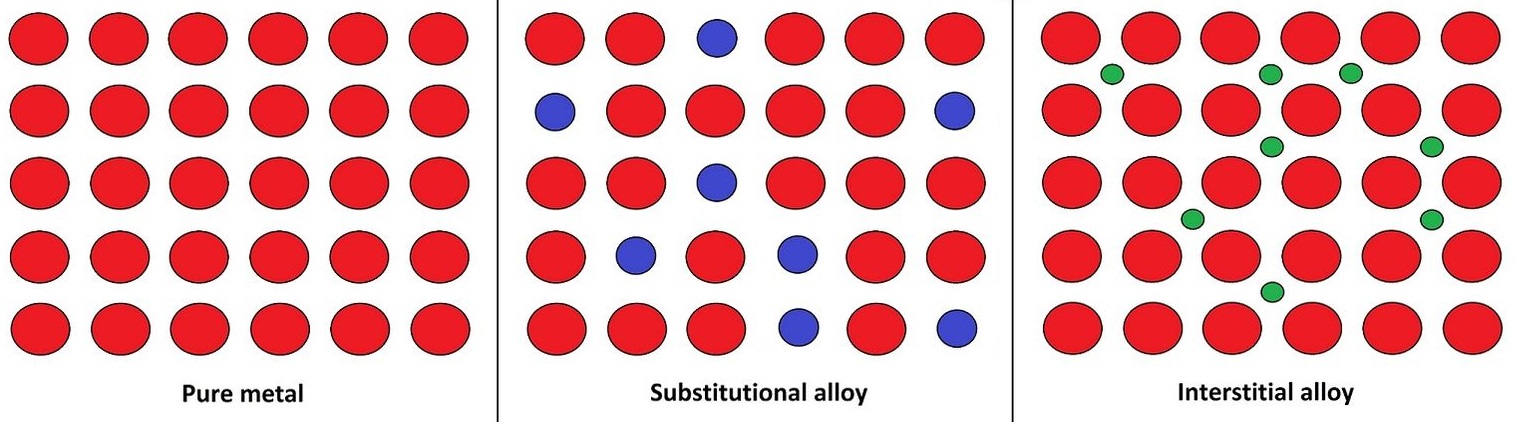
Metals can be made into alloys.
- interstitial alloys occur when there are two metals with vastly different radii combine. Steel has large iron atoms and small carbon atoms.
- substitutional alloys occur when metals of similar radii combine. Brass has copper and zinc, both similar sizes.
Molecular Covalent Bonding
In covalent bonds, two nonmetals share electrons in the valence shell of the two atoms.
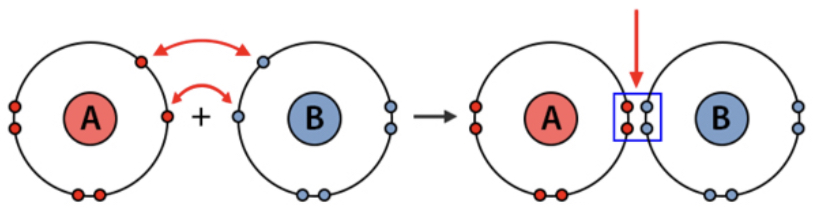
When two or more atoms bond covalently, they create a molecule, which can be as small as two atoms or even as large as 24, there's no size limit.
The first covalent bond between two atoms is called a sigma bond. all single bonds are also sigma bonds. The second bond, a double bond, is called a pi bond. The second and third bond in a triple bond is a pi bond. Double and triple bonds are shorter and stronger than single bonds, but not double or triple the strength,
Internuclear Distance
The length of a covalent bond depends on balancing the attractive and repulsive forces. when two atoms are too close, the potential energy is high, and the nuclei reset each other. When they are too for, the potential energy is zero because they cannot attract.
\n
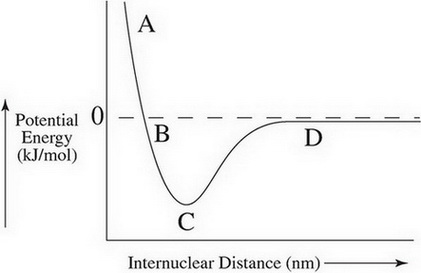
The dashed line represents zero potential energy, but it can be negative, which represents lower energy. at label A, atoms are too close to bond. at label D they are too far. at label C the repulsive and attractive forces are equal and they can bond.
Network Covalent Bonds
- Network covalent bonds as solids are held together in a lattice of covalent bonds which makes them very hard, very high melting point and boiling point. Electrons cannot more about the lattice, making them poor conductors.
- The most common network solids are carbon (graphite or diamond) and silicon (quartz) because of the four valence electrons that allows more covalent bonding.
Conductivity
- The type of bonding can be determined by looking at if it's a good conductor of electricity. this chart shows conductivity based on phase
| Solid | Aqueous | Liquid | Gas | |
|---|---|---|---|---|
| Ionic | No | Yes | Yes | No |
| Molecular covalent | No | no | no | No |
| Network covalent | No | n/a | No | no |
| Metallic | Yes | N/A | yes | No |
- Covalent substances never conduct electricity, including pure water. Tap water is not pure water and is filled al dissolved ions that can conduct.
- Ionic substances cannot conduct as solids because of the electrons stuck in the lattice structure. As liquids and when aqueous, the electrons can move around and conduct. The ability to conduct depends on concentration and now many ions they dissociate into.
- 1 M of NaCl conducts better than 0.1 M of NaCl because there's more ions present per unit of solution.
- however, 1 M of CaCl2 would conduct better than NaCI because it dissociates into three ions rather than two ions.
Lewis Dot Structures
Drawing Lewis Dot Structures
- Steps to drawing Lewis Dot Structure
- Using the periodic table, count the valence electrons in the molecule
- if it has a neg charge, add the amount of electrons equal to the charge. If it's positive, subtract
- draw skeletal structure of molecule with the most electronegative in the middle and draw a pair electrons (single bond) between the elements.
- Add electrons to each element until there's a complete outer shell.
- add remaining electrons to central atom.
- if central atom has less than eight electrons, remove pair from outer atom and make it a bond
- if it's a complete octet, you're finished
Resonance Forms
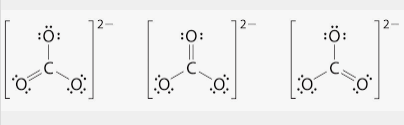
- The Lewis dot structure of CO3 can be drawn with 2 single bonds and one double bond on any oxygen. Despite there being a double bond, each bond is a similar strength and length, in between a double and single bond.
- To calculate the relative strength and length, you can use bond order calculations.
- a single bond has a bond order of one and a double bond has a bond order of two. Add up the amount of single and double bonds and divide by now many resonance forms there are.
- (1+2+1)/3= 1.33
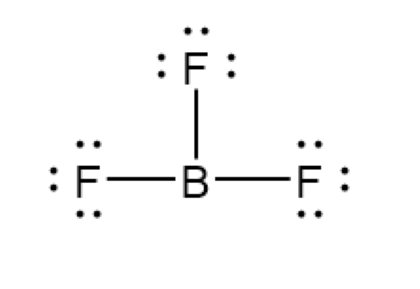
Incomplete Octets
Some atoms do not need eight valence electrons. Hydrogen and helium only need two, but helium never bonds. Boron is stable with six electrons. All others need eight electrons in covalent bonds.
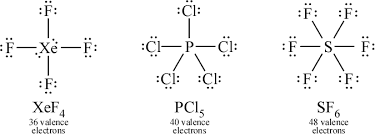
Expanded Octets
Molecules with d subshells can have more than eight valence, but never more than twelve. Silicon, phosphorus, sulfur, and chlorine can expand, but never carbon, oxygen, nitrogen. Noble gases can sometimes bond because of their empty d-orbital.
Formal Charge
Even though a molecule has several variations a Lewis dot structures, there is a more likely structure, called formal charge. To find formal charge, subtract the total number of valence electrons from the number of assigned electrons (bonded and lone pairs).
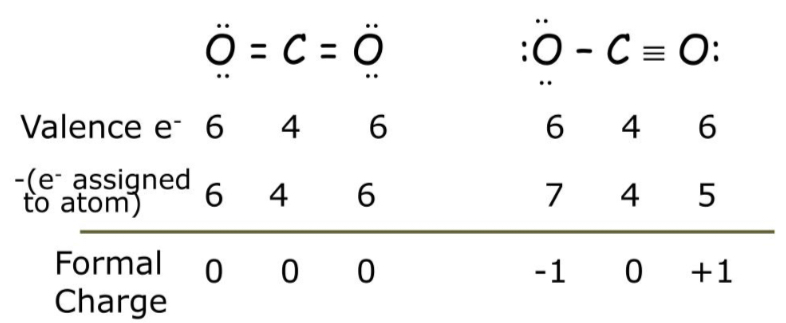
To determine which structure is more likely, choose the one with the formal charge of zero (the left structure on the diagram).
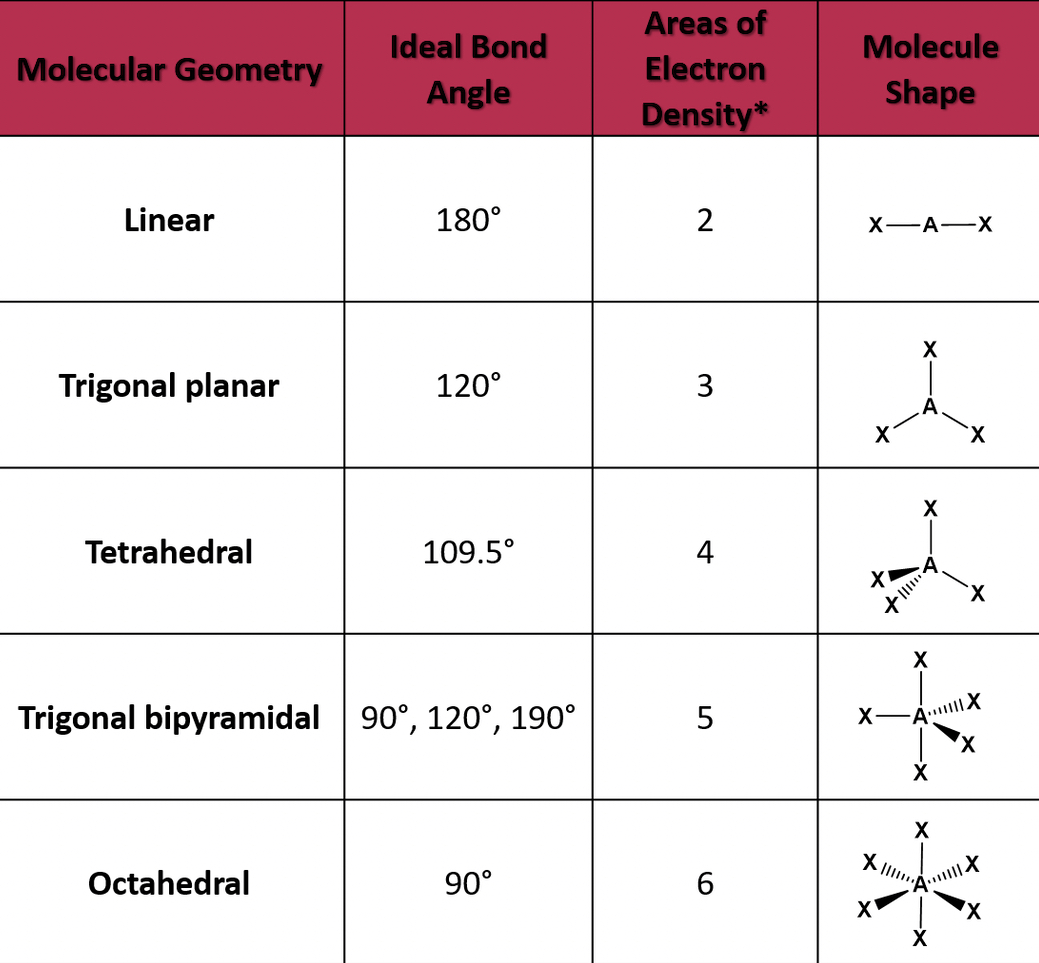
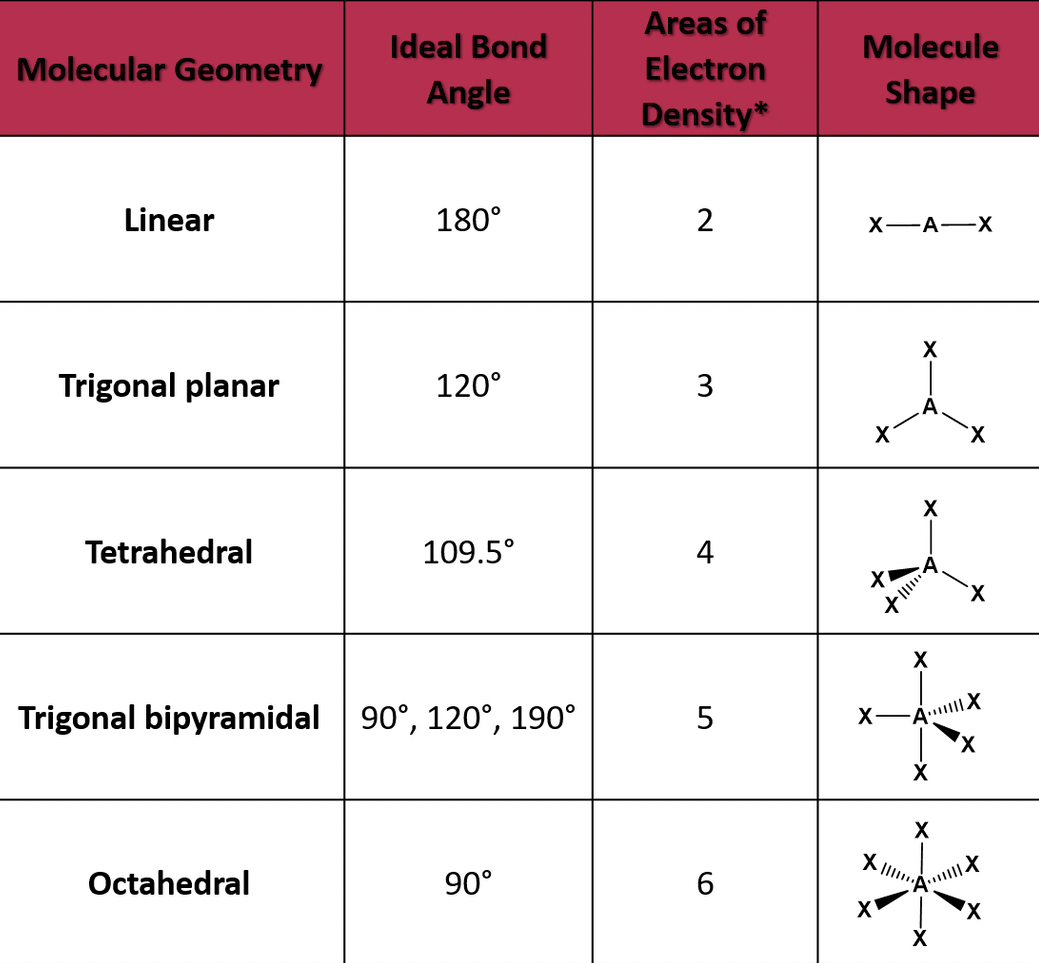
Molecular Geometry
Molecules will arrange in different shapes to keep electron pairs as far apart as possible to limit repulsion. To determine the shape, the valence shell electron pair repulsion model is used (VESPR).
When there's more than two electrons, the share depends on the number of bonds and lone pairs on the central atom.
- Double and triple bonds are treated the same in terms of predicting overall geometry for a molecule
- Lone electron pairs have a more repulsive strength than bonds.
Types of geometries
- If the central atom has two electron pairs, then it's an sp hybridization with a linear shape.
- If the central atom has three electron pairs, it has the sp2 hybridization, and a basic shape of trigonal planar
- The central atom has four electron pairs, it has an sp3 hybridization and a basic tetrahedral shape.
- The central atom has five electron pairs, it has a trigonal bipyramidal shape.
- The central atom has six electron pairs, its octahedral.