Unit 7: Equilibrium
The Equilibrium Constant, Keq
In equilibrium reactions, the reaction is reversible, where the reactants from products and then go back to reactants constantly.
- In the Haber process, the industrial production of ammonia, N2+3H2 ⇌ 2NH3, the reaction is reversible.
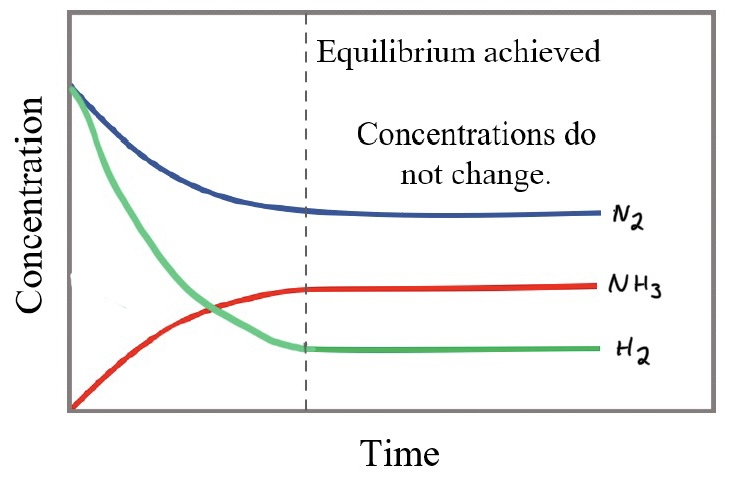
- At the equilibrium point, the amount of reactants and products has stopped changing, but the reaction is still occurring, the forward and reverse reactions are equal.
- The coefficients determine at what rate a substance is created or consumed. The H2 is consumed three times faster than the N2, so it is a steeper slope when they are initially the same concentration.
- The Law of Mass Action is the equilibrium expression that determines the relationship between reactant and product concentrations.
- Equilibrium expression for the reaction aA+bB ⇌ cC+dD
- Keq = [C]^c[D]^d / [A]^a[B]^b
- [A], [B], [C], [D] are the molar concentrations or partial pressures at equilibrium
- Divide the products by the reactants
- Only gasses and aqueous solutions are in the equilibrium expression
- No units for Keq
- A Keq > 1 means the products are favored over reactants. A Keq < 1 means reactants are favored over products.
- Different types of K values, or equilibrium constant
- Kc is constant for molar concentrations
- Kp is partial pressure constant
- Ksp is solubility product constant, which has no denominator because reactants are solids
- Ka is the acid dissociation constant
- Kb is base dissociation constant
- Kw is the ionization of water (Kw is 1.0 x 10^-14)
Manipulating Keq
- Hess’s law can be applied to equilibrium equations
- If a reaction is flipped, take the inverse of the equilibrium constant
- If you multiply the coefficients, take the constant to the power (multiply by two, square the constant)
- If you add reactions together, multiply the constants together to get the new constant
Le Chatelier’s Principle
- Whenever a stress is placed on a system, the reaction will shift to go back to equilibrium. If a reaction shifts left, it will create more reactants. If it shifts right, it will make more products.
Concentration
- If concentration is altered, the reaction will shift to use up more or less of the substance with an altered concentration.
- For the Haber process, if N2 or H2 is added, it will shift right to create more NH3 and use up more of the reactants. If more NH3 is added, the reaction will shift left to create more N2 and H2.
- If N2 or H2 is removed, it will shift left to make up for the loss of the reactant and create more reactants.
Pressure
- When external pressure is increased, the partial pressure of gases inside a container will increase, and the reaction will shift to the side with less gas molecules. Decreasing the partial pressure will cause the reaction to shift to the side with more gas particles
- If the pressure of a container is increased, the reaction will shift to the right where there are 2 moles of gaseous products rather then the left, where there are four moles of gaseous reactants.
- If the pressure is decreased, the reaction will shift left where there’s more moles of gas.
- If a noble gas is added at a constant pressure, the equilibrium reaction will shift to the side with more gaseous molecules. If a noble gas is added to a reaction at a constant volume, no shift occurs.
Temperature
- The Haber process has a ΔH of -92.6 kj/mol, so it is exothermic, so heat is generated, this creates the equation:
- N2+3H2 ⇌ 2NH3 + energy
- If the temperature goes up, the reaction will shift to the left, making more reactants. If the temperature goes down, the reaction would shift to the right and produce more products
Dilution
- If an aqueous equilibrium is diluted, such as the equation Fe 3+ (aq) + SCN- (aq) ⇌ FeSCN 2+ (aq), diluting an equation with extra water will cause a shift.
- If this equation was diluted, it would shift the side with more aqueous molecules, the left. If water evaporated, it would shift to the side with less aqueous molecules, or the right.
Changes in the Equilibrium Constant
- When concentration or pressure changes, the equilibrium constant stays the same and the product to reactant ratio will revert to the original value.
- When temperature is changed, the equilibrium constant is changed and will be a new value because it affects the kinetics and the equilibrium constant.
The Reaction Quotient, Q
- The equilibrium constant can only be used when equilibrium is achieved, but the reaction quotient, Q, can be used at any point in time during a reaction to determine how close or far the reaction is to equilibrium and how it will shift.
- The equation is the same as the Keq equation.
- If Q is less than K, the reaction shifts right.
- If Q is greater than K, it will shift left
- If Q and K are equal, the reaction is at equilibrium.
Solubility
- A salt is considered soluble if one gram of the salt is dissolvable in 100 mililiters of water. Soluble salts are assumed to have completely dissolved in an aqueous solution. Most solids increase in solubility as the temperature increases.
Solubility Product (Ksp)
- Ksp is the measurement of how much a salt dissolves in a solution. The larger the Ksp value is, the more soluble the salt is.
- For the reaction AaBa (s) ⇌ aA (aq) + bB (aq)
- The solubility expression is Ksp= [A]^a[B]^b
- The solubility of a salt is equal to the ion concentration it dissolves into.
- If the molar solubility of the salt AaBa is 1.3x10^-4 M, then the A ion concentration is the same as the solubility time the value a. (Keep in mind the salt to ion ration)
- Typically, as temperature increases, the increase in molecular motion allows the salt to dissolve more, but sometimes it will decrease as temperature increases. This is hard to predict.
The Common Ion Effect
- If AgCl is put into a solution to dissolve, it will only do so in small amounts. If NaCl is added to the solution, it will dissolve completely and not affect the Ag ions. The Na ions can be ignored but all of the Cl ions from the NaCl and AgCl must be considered because of the common ion effect.
- According to the common ion effect, the new Cl ions will affect the AgCl equilibrium even though the Cl ions came from NaCl.