Unit 4: Chemical Reactions
Types of Reactions
- Synthesis reaction: when elements or simple compounds are combined to form a single, more complex compound. A+B → AB
- Decomposition: opposite of synthesis-occurs usually in the presence of heat. AB → A + B
- Acid-base: when an acid (H+) reacts with a base (OH-) to form water and a salt. HCl (aq) + NaOH (aq) → NaCI (aq) + H2O (l)
- Oxidation-reduction (redox): results in change of oxidation state (only for some). Cu 2+ (aq) + 2 e- → Cu (s)
- Hydrocarbon combustion: carbon + hydrogen (and sometimes oxygen) is ignited and produces CO2 and water, sometimes other compounds as well depending on the elements present. C4H10 + O2 → CO2 + H2O
- Precipitation: two aqueous solutions mixing to create a solid precipitate. A (aq) + B (aq) → C (aq) + D (s)
- Some solubility rules:
- Compounds with alkali metals and ammonium are always soluble.
- Compounds with nitrate are always soluble.
Chemical Equations
Balancing Chemical Equations and Calculations
- Stoichiometry steps:
- Covert given quantity to moles → use equation coefficients to determine limiting reactant → use balanced equation to determine how much product generated → convert moles to desired unit.
Percent error
- % error = (|experimental value - expected value| / expected value) x 100%
Combustion Analysis
- Because of the law of conservation of mass, when a hydrocarbon is combusted, all of the carbon in the hydrocarbon will create CO2 and all of the hydrogen will end up as H2O.
Gravimetric Analysis
Gravimetric analysis is used to determine an unknown element in a precipitate reaction.
A 4.33 g sample of an unknown alkali hydroxide compound is dissolved completely in water. A sufficient solution of copper (IL) nitrate is added to the hydroxide solution such that it will fully precipitate copper (Il) hydroxide via the following reaction: Cu 2+ (aq) + 2 OH- (aq) → Cu (OH)2 (s). After the precipitate is filtered and dried, its mass is found to be 3.81 g. Is the original alkali hydroxide sample most likely LiOH, NaOH. or KOH?
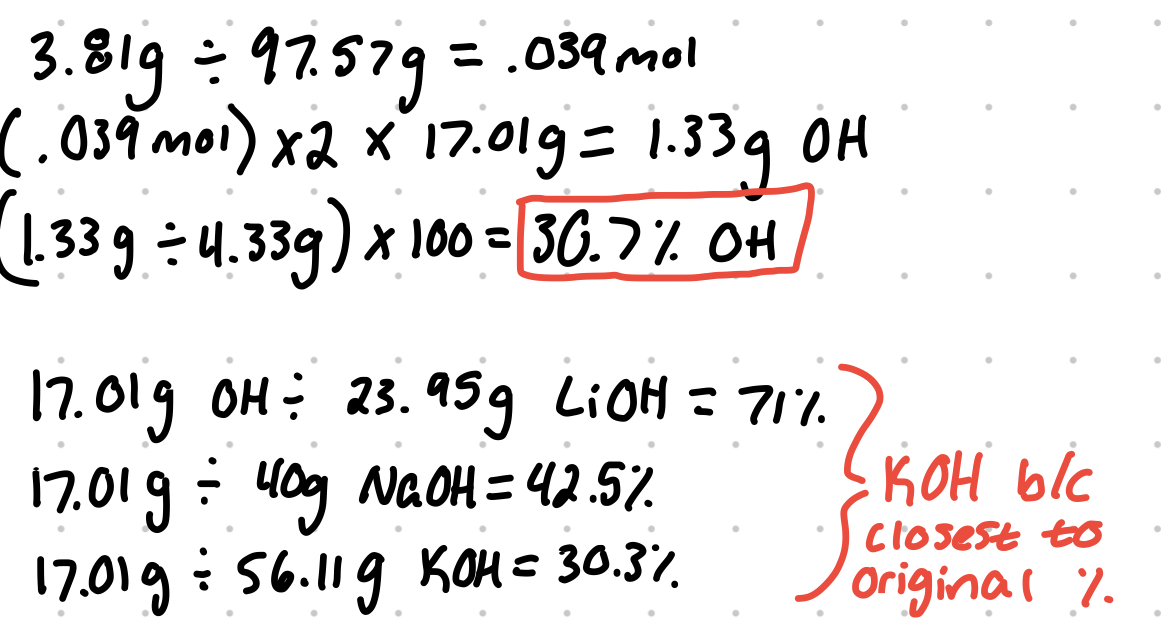
Oxidation States
- In chemical reactions, electrons are transferred between reactants and can be determined with oxidation states, which have several rules.
- Any neutral atom not bonded has an oxidation state of zero.
- A singular atom has an oxidation state equal to it's charge.
- In most compounds, oxygen is -2. One exception is H2O2, where oxygen is -1
- When bonded to a nonmetal, hydrogen is +1. When bonded to a metal, hydrogen is -1.
- When oxygen is not present, the most electronegative element has a state equal to it's most common charge.
- The combined oxidation states must be equal to the charge of that compound
Oxidation-Reduction Reactions
- In redox reactions, electrons are swapped between reactants and the oxidation states of the reactants are changed.
- In Fe + 2 HCL → FeCl2 + H2, iron's state goes from 0 to +2 and H goes from +1 to 0.
- Fe was oxidized
- It can be written as a half reaction
- Fe → Fe 2+ + 2e- oxidation
- 2 H+ + 2e- → H2 reduction
Redox Titrations
- To determine the concentration of an unknown solution, a chemical added until usually a color change occurs.
- If KMnO4 is added to a solution with MnO4 (which is a deep purple), the Mn is reduced and a color change occurs. If KMnO4 is added to a colorless solution that can be oxidized, it will turn pink.
Acids and Bases
- An acid is a substance capable of donating a proton (H+) and a base is considered a substance capable of accepting a proton.
- HC2H3O2 + H2O ⇌ C2H3O2- + H3O-
- — HC2H3O2 and H3O are acids while H2O and C2H3O2 are bases
- The H+ ion is the acid and the one without is the base. These are called conjugate pairs
- HC2H3O2 and C2H3O2-
- H2O and H3O-
- However, water can sometimes act as an acid, this is called amphoteric