Unit 6: Thermodynamics
Heat and Temperature
- Temperature is the average amount of kinetic energy based on molecular motion of a substance
- Heat is the flow of energy between two substances at different temperatures
- The first law of thermodynamics states energy cannot be created or destroyed and can only transfer or change forms. Energy transfers through molecular collisions.
- Exothermic is when energy is transferred from the system to the surroundings, such as when a chemical reaction occurs in a beaker and the beaker feels hot. Endothermic reactions occur when a system absorbs the energy from the surroundings, such as when a beaker feels cold.
Enthalpy
Enthalpy Change, ΔH
- Enthalpy change is the measure of energy that is released or absorbed when bonds are broken/formed
- When bonds are formed, energy is released. When bonds are broken, energy is absorbed.
- If more energy is released than absorbed in a reaction, then it is exothermic and has negative enthalpy change. If more energy is absorbed than released in a reaction, then it is endothermic and has a positive enthalpy change.
Energy Diagrams
Exothermic and Endothermic Reactions
When products have a lower energy than the reactants, the ΔH is negative and it is an exothermic reaction
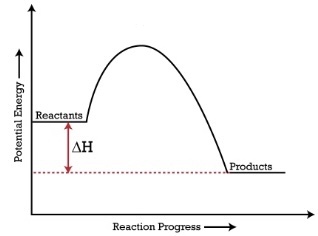
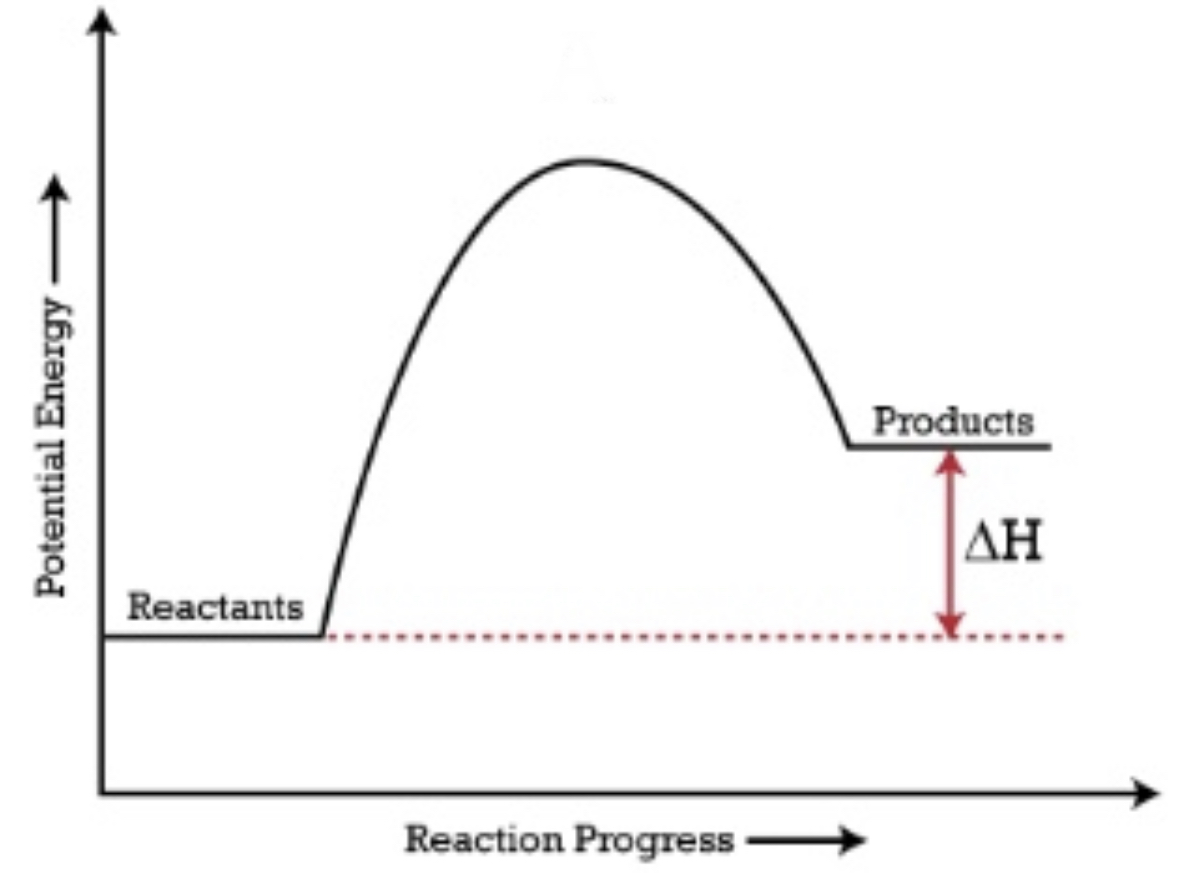
When reactants have a lower energy than the products, the ΔH is positive and it is an endothermic reaction
Catalyst and Energy Diagrams
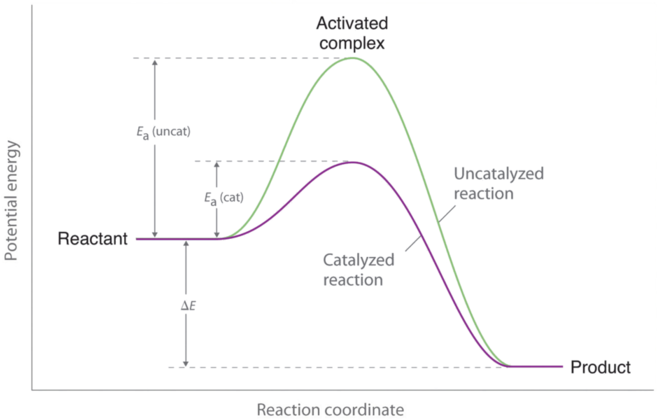
- Catalysts speed up a reaction by lowering the required activation energy and only changes the activation energy, not the amount of energy of products or reactants in the end or beginning (ΔH).
- This change can only occur when a reaction is not in equilibrium conditions
Enthalpy of Formation, ΔHf°
- Enthalpy of formation, or heat of formation, is the energy change when one mole of a compound is formed from pure elements under standard conditions (298°K, 1 atm)
- Example: C(s) + 1/2 O2 (g) + 2 H2 (g) → CH3OH
- There is exactly one mole of a product, methanol, which has an odd number of elements, and diatomic in nature.
- ΔHf° of a pure element is defined as zero and stands true for elements that are diatomic in pure states, such as hydrogen or oxygen.
- If ΔHf° of a compound is negative, energy is released when the compound is formed from pure elements, is exothermic, and is more stable than the reactants.
- If ΔHf° is negative, the opposite is true, and it is endothermic.
- ΔH can be found if ΔHf° of product and reactants are known
- ΣΔHf° Products - ΣΔHf° Reactants = ΔH
- The units for reactions enthalpy is kj/mol.
Bond Energy
- Bond energy is the energy required to break a bond and is always a positive number. When a bond is formed, the energy of formation is equal to the bond energy released.
- ΔH = Σ Bond energy of bond broken - Σ bond energy of bonds formed
- Bonds broken are the reactant bonds and the bonds formed are the product bonds
- The number of bonds broken is determined by how many moles of that substance there is and how many individual bonds there are.
- For 2 H2O molecules, there are two H-O bonds and two of the H2O molecules. So, there are four H-O bonds and to find the bond energy, multiply four by the bond energy of H-O (463 kJ/mol).
Hess’s Law
- Hess’s Law states that if a reaction can be broken into a series of steps, the sum of the ΔH of those steps are equal to the ΔH of the reaction.
- When manipulating equations for enthalpy calculations…
- If a reaction is flipped, flip the enthalpy value (pos to neg, or neg to pos)
- If you multiply or divide the coefficients of a reaction, multiply or divide the enthalpy value by that same interval
- Adding up the enthalpy values of steps will be the new enthalpy value for the new reaction created from those steps
Enthalpy of Solution
- When an ionic substance dissolves in water, energy is absorbed because bonds are breaking, but energy is also emitted because new dipoles are forming.
- Step one of dissolving: Bonds are broken in the solute. This step has a positive ΔH because bonds are being broken
- Step two: solvent molecules are separated. The water molecules are spread apart to make room for the solvent ions. This weakens intermolecular bonds, resulting in a positive ΔH
- Step three: New attractions are created. The free-floating ions are attracted to the dipoles in the water molecules, resulting in a negative ΔH despite no bonds being formed.
- Step two and three values are combined to form the hydration energy which is always a negative number. Hydration energy is a Coulombic energy and it’s magnitude increases as the ions increase their charge or decrease the size.
- If adding all three steps together, the enthalpy of solution can be determined.
Thermodynamics of Phase Change
Naming the Phase Changes
- Solid to liquid: melting
- Liquid to solid: freezing
- Liquid to gas: vaporization
- Gas to liquid: condensation
- Solid to gas: sublimation
- Gas to solid: deposition.
- Vapor pressure is the amount of pressure given off by molecules as they go from solid/liquid to gas.
Enthalpy of Fusion
- Enthalpy of fusion is the amount of energy required to cause a solid to melt. Heat of fusion is how much heat given off when a substance freezes. The intermolecular forces in a solid substance are more stable when they are solid so they have a lower energy than liquid forces, so energy is released in the freezing process.
Enthalpy of Vaporization
- Enthalpy of vaporization is how much energy is required to turn a liquid to a gas. The heat of vaporization is the energy given off when a gas condenses. Intermolecular forces are more stable in a liquid state, so energy is released as the forces stabilize and get stronger.
- When heat is added to a substance, it will either phase change or increase in temperature, not both. Only one can happen at a time. Temperature remains constant during a phase change.
Calorimetry
- Calorimetry is the measurement of heat changes during a chemical reaction
- The specific heat of substance is how much heat is required to raise one gram of a substance by one degree celsius (or one degree kelvin). The higher or larger a specific heat is, the more heat a substance can absorb without changing temperature. A low specific heat means less heat is required to change the temperature of a substance.
- Specific heat equation is q = mcΔT
- q is heat added (J or cal)
- m is the mass of the substance (g or kg)
- c is specific heat
- ΔT is temperature change (K or C