MD 1. Intro to Carbonyls
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
What is the highest priority carbonyl group when naming?
Carboxylic acid
priority for carbonyl naming is carboxylic acid, ester, acyl chloride, amide, aldehyde, ketone
|So if the compound has a carboxylic acid group and a ketone it would have the -oic acid suffix, with an oxo- prefix for the ketone.
2
New cards
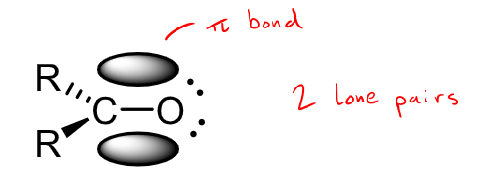
What hybridisation does the carbonyl group have?
SP2 hybridisation - 3 sigma bonds and a pi bond
3
New cards
Is the carbonyl a planar group?
yes and it follows a trigonal planar shape with 120 degree angles.
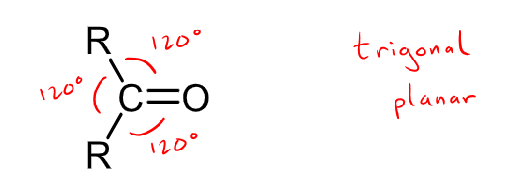
4
New cards
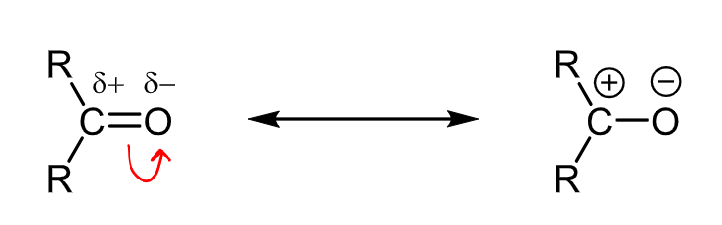
What are the dipoles and resonance forms of the carbonyl group?
5
New cards
What is the typical NMR shift for an aldehyde?
H NMR - shift at 9-10ppm
C13 NMR - shift at 165-205ppm