MD 2. Carbonyl Reactivity and General Reactions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
How do nucleophiles attack carbonyls?
attack the partially positive carbon and can attack from either face of the planar molecule.
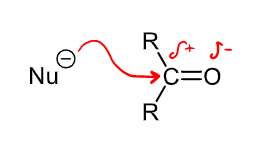
What is the general mechanism for a nucleophilic addition attack on a carbonyl? What is an example of this reaction type?
This could be the reduction of an aldehyde to a primary alcohol, H- is the nucleophile that bonds to the carbon, followed by a work up step where H+ reacts with the negatively charged oxygen.
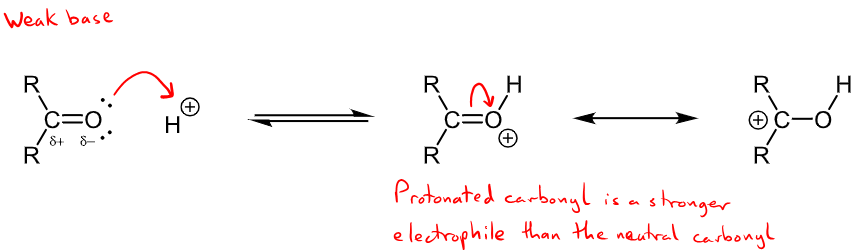
How do electrophiles attack carbonyls? Under what conditions?
occurs in acidic conditions so there is excess H+
Electrophile attacks the partially negative oxygen - not a common reaction
What electron effect is on the carbonyl group that explains why the hydrogens at the alpha position are acidic?
the electron withdrawing, -M mesomeric effect, causes an enolate ion to be formed when the alpha hydrogens are lost.
The enolate ion is a stronger nucleophile than the neutral carbonyl
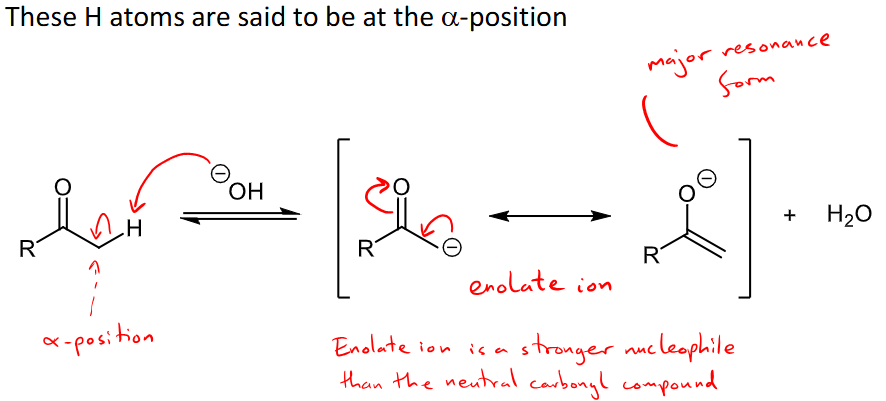
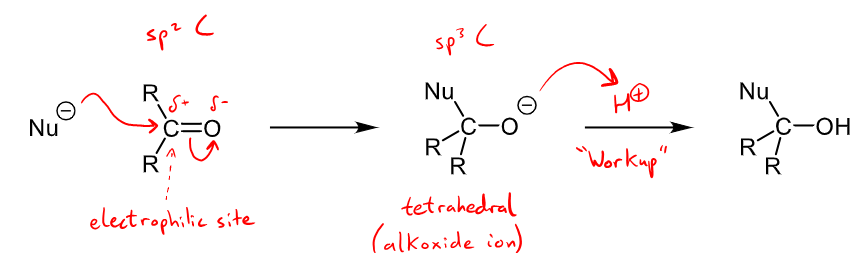
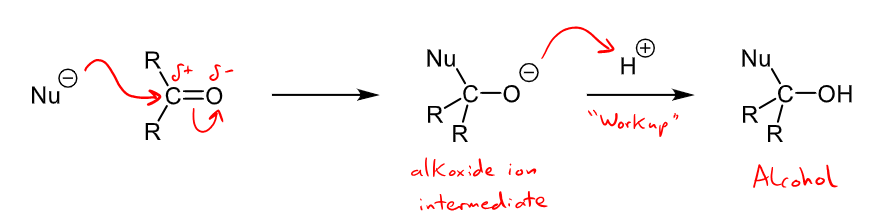
What are examples for charged nucleophiles and their addition mechanism when reacting with aldehydes and ketones?
charged nucleophiles include H-, R-, CN-
alcohol is formed with an alkoxide intermediate
Nucleophilic addition occurs commonly with aldehydes and ketones
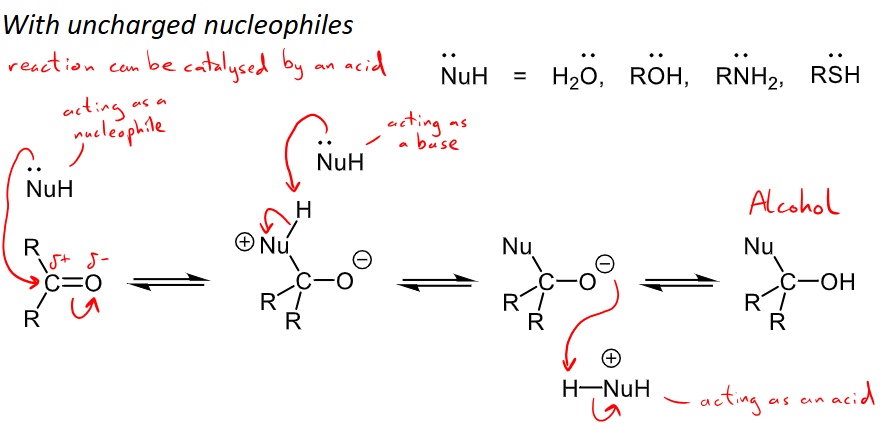
What are examples for uncharged nucleophiles and their addition mechanism when reacting with aldehydes and ketones?
uncharged nucleophiles = H2O, ROH, RNH2, RSH (the O, N or S all have a lone pair that are able to react or act as the nucleophile)
Reaction can be catalysed by an acid
Nucleophilic addition occurs commonly with aldehydes and ketones
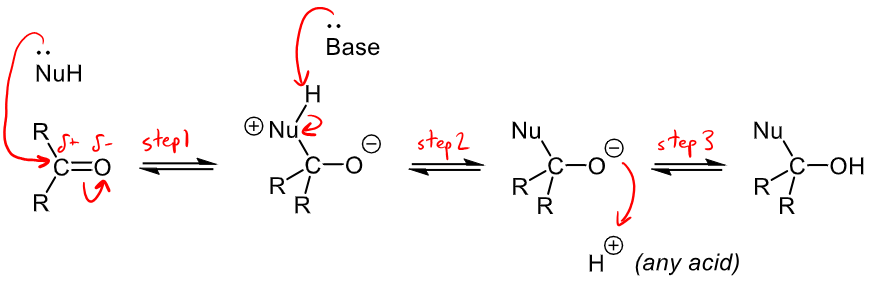
What are the proton transfer steps in the uncharged nucleophilic addition mechanism and what compounds can aid these proton transfers?
in step 2 a proton is removed from the nucleophile that is reacting, this can be removed by a base within the reaction mixture
In step 3, a proton is donated to form the final alcohol group, this can be donated by any acid in the reaction mixture.
What is the charged nucleophilic substitution mechanism? What carbonyls and leaving groups does this occur with?
Occurs with carboxylic acid derivatives: acyl chlorides, acid anhydrides, esters, amides (does NOT occur for aldehydes/ketones)
Leaving group, X, could be Cl, OCOR, OR or NR2
charged nucleophile = H- or R-
nucleophile attacks partially positive carbon, unstable alkoxide is formed where leaving group is kicked off and then product is formed with leaving group by product.
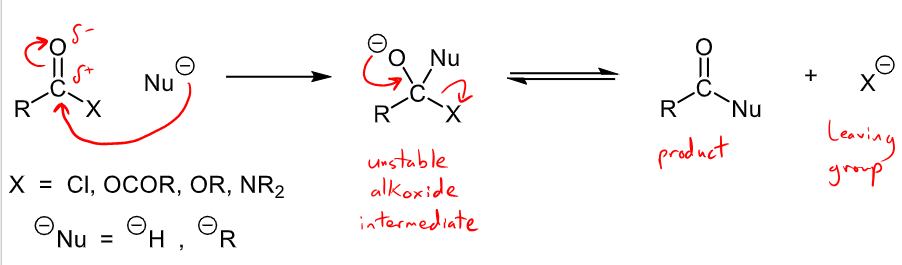
What is the uncharged nucleophilic substitution mechanism? What carbonyls and leaving groups does this occur with?
Occurs with carboxylic acid derivatives: acyl chlorides, acid anhydrides, esters, amides (does NOT occur for aldehydes/ketones)
Uncharged nucleophiles = H2), ROH, R2NH, RSH (all have a lone pair that react with the C)
X = Cl or OCOR
Nucleophile lone pair attacks partially positive carbon, base then removed hydrogen from nucleophile, then leaving group is kicked off.
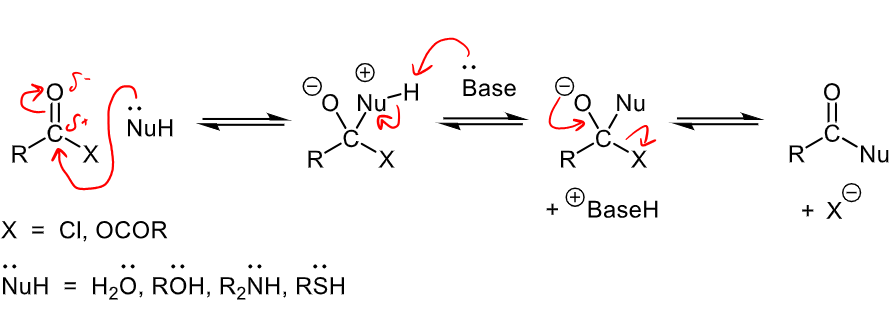
What are the two routes carbonyls can go through for an alpha substitution reaction?
The molecule tautomerizes and becomes and enol, and is then attacked by ana electrophile and loses a proton.
A negative charged base is added, taking off a proton to form an enolate ion, then it is attacked by an electrophile.
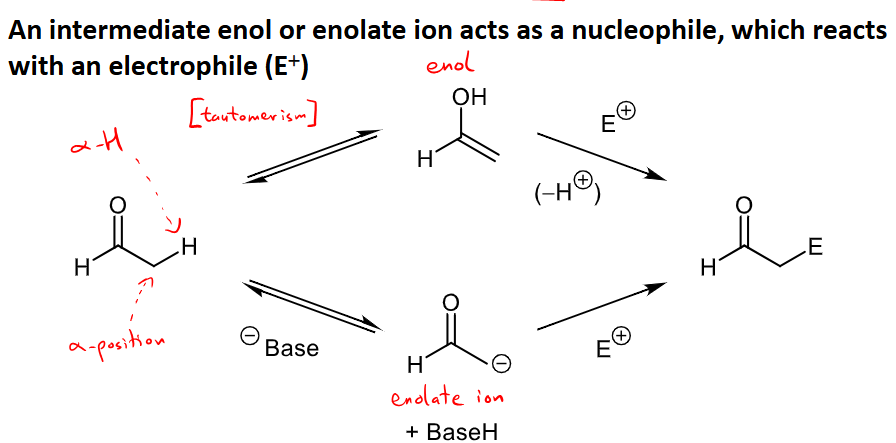
What is carbonyl-carbonyl condensation and its conditions and mechanism for ketones and aldehydes?
two carbonyls reacting to form a product with anew carbon-carbon bond
two aldehydes or two ketones react, one undergoes alpha substitution and the other undergoes nucleophilic addition.
H2O is eliminated making it a condensation reaction.
E.G Aldol condensation - two aldehydes reacting.
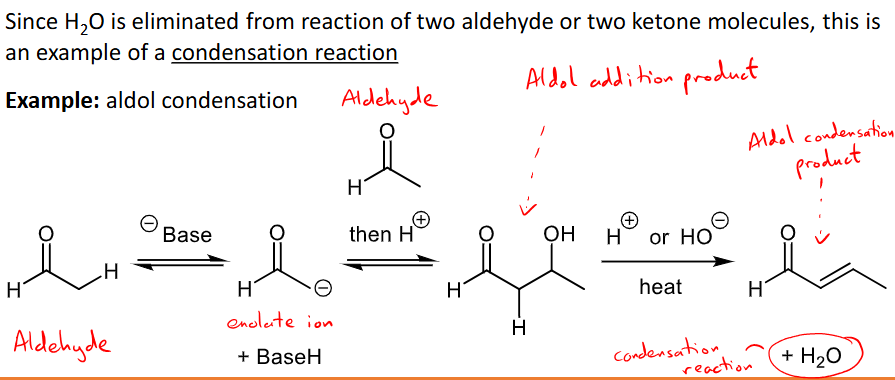
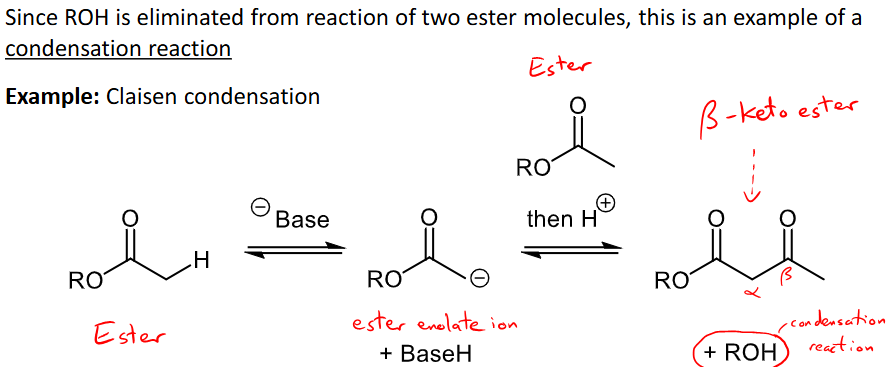
What is carbonyl-carbonyl condensation and its mechanism for carboxylic acid derivatives?
2 carboxylic acid derivatives (esters etc), one undergoes alpha substitution and one undergoes nucleophilic acyl substitution
ROH is eliminated making it a condensation reaction.
E.G Claisen condensation - ester reacts with base to make enolate ion (alpha substitution), then another ester and H+ is added to form a keto ester by ROH by-product.