Ochem Reagents
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Zipper Reaction
- KNH₂/NH₃
- H₂O
Allylic Halogenation
- NBS/NCS, CCl₄, initiator, heat (∆)/light (hv)
Diels Alder
- Heat (∆)
Free radical bromination (benzylic)
- NBS, CCl₄, initiator, heat (∆)/light (hv)
Protection example
- NaH/DMSO
- Ph-CH₂-Br
Birch Reduction
- Li or Na, liquid NH₃
- Alcohol (i.e. ethanol)
Alkyne Reduction
- Pd/C/BaSO₄
Sulfonation
- SO₃/H₂SO₄
Nitration
- Conc. H₂SO₄, conc. HNO₃
Bromination
1.Br₂/FeBr₃
Chlorination
- Cl₂/AlCl₃
Friedel-Crafts Alkylation
- R-Cl, AlCl₃, CS₂ (solvent)
Friedel-Crafts Acylation
- R-COCl, AlCl₃ (>1 eq.), CS₂ or CH₂Cl₂/DCM (solvent)
- H₂O
Clemmensen Reduction
- Zn/Hg/HCl
Wolff-Kishner Reduction
- H₂N-NH₂, KOH (base), Diglyme or ethylene glycol (solvent), 200°C
Reverse FC Alkylation
- H₂O/H⁺, heat(∆)
NO2 Reduction
- Fe/HCl OR Sn/HCl OR Pd/C/H₂
Diazotization
- NaNO₂, HCl, 0-5°C
Sandmeyer Reaction
- CuBr OR CuI OR CuCN, Diazonium Chloride
Benzene Regeneration (From Diazonium Chloride)
H₃PO₂
Oxidation
KMnO₄/H₂O/heat OR K₂Cr₂O₇/H₂SO₄/heat
Iodobenzene Formation
KI, Diazonium Chloride
Phenol Formation
H₂O, heat(∆), Diazonium Chloride
Reverse Sulfonation
H⁺/H₂O, heat (∆)
Benzene to cyclohexane (catalytic hydrogenation)
H₂/Pt, ethanol or acetic acid (polar solvent), pressure
Amide hydrolysis
NaOH, ∆
Amine to Nitro Conversion
O₃
CN Oxidation
H₂O/H₃O⁺, ∆
Elimination-Addition (Benzyne production)
300°C, strong base/nucleophile
Chichibabin Reaction
1.Pyridine, KNH₂, 1-4-dioxane, ∆
- H₂O
Addition of CN
Base catalyzed (usually), i.e pyridine
Acetal/Ketal Formation
Excess alcohol/acid catalyst
Reverse acetal/ketal formation
H⁺, H₂O, ∆
Carbonyl compounds with amines
pH 4-5
Balyer-Villiger Oxidation
F₃C-CO₂-OH
Oxidation (past aldehyde)
CrO₃, H₂O, H⁺u
Oxidation (stop at aldehyde)
PCC (Pyridinium Chlorochromate) in CH₂Cl₂, rt OR Pyridine CrO₃ complex
Moffat-Swern Oxidation
- Alcohol, DMSO, oxalyl chloride, CH₂Cl₂, -78°
2.Et₃N, -20°
Protecting alcohols with acetals
Dihydropyran or Dihydrofuran, excess H⁺
CN to CH2NH2
- LiAlH₄, ether, heat
- Workup
Organometallic reactions (RMgBr, RLi, R2CuLi)
Ether
NaBH4
Alcohols, acids, or any solvent with a pKa <20
LiAlH4 solvent
Ether or THF (Non-protic solvent)
Oxidative Cleavage of Diols
HIO₄, H₂O
Disulfide formation
Br₂, NaHCO₃(base), RSH
Sulfoxide formation (and water)
RSR, H₂O₂
Sulfone formation
F₃C-CO₂-OH
Ozanolysis
- O₃/MeOH
2.Me₂S
Wittig Reaction (Phosphonium Ion Formation)
Ph₃P, CHCl₃, rt
Wittig Reaction (Carbanion/Alkene formation)
BuLi, THF
Dihydroxylation of alkenes (Formation of a diol)
- OsO₄/Pyridine
2.H₂S
Aldehyde to Carboxylic Acid (oxidation)
NaClO₂
Alkene Oxidation
1.O₃/CH₃OH/CH₂Cl₂
2.H₂O₂
Carboxylic Acid formation from organometallic reagents
1.CO₂
2.H₃O⁺/H₂O
Carboxylic Acid formation from nitriles
1.H₂O/OH⁻ Na⁺
- H₃O⁺
Fisher Esterification (forward)
cat. H⁺
Fisher Esterification (reverse)
Excess H₂O, ∆
Acid Anhydride formation
Carboxylic acid/carboxylate, acid chloride
Ester synthesis
- CsOH/KOH (strong base)
2.DMSO, ∆, R-Br
Methyl Ester Synthesis
Diazomethane (does not react with alcohols and amines)
Acid chloride formation from acids
SOCl₂ or PCl₃ or PCl₅
Thioester formation from acid chlorides
M⁺ ⁻SR
Ester formation from acid chlorides
ROH, base
Amide formation from acid chlorides
RNH₂, base, rt
Carboxylic acid to amide (compatible with water)
DCC
Carbox. acid to ketone (organometallic)
R-Li, ether
Anhydride formation
2 carboxylic acids, (-H2O), heat, >200
Addition-Elimination
NaOH or NaOR, rt
H2O
Addition of Alkyl Lithium
R-Li, ether
H2O work up
Ester —> Aldehyde
DIBAL-H
Amides and LiAlH4
R-CH2-NH2
Nitrile —> aldehyde
DIBAL-H
Nitrile to ketone
R-MgBr or R-Li
H3O+
Ketene to amide
R-NH2
Baeyer-Villiger Oxidation of Ketone
mCPBA, H⁺, CH₂Cl₂ (3° R migrates best)
Beckmann Rearrangement
1) H₂N-OH (to oxime)
2)H₂SO₄ or H₃PO₄ (strong acid), heat
Arandt-Eistert/Chain elongation (First Step)
CH2N2
Arandt-Eistert/Chain elongation (Second Step, Wolff Rearrangement)
H2O
Curtius Rearrangement
acid chloride, NaN3, H2O
LDA, THF
Least substituted alpha hydrogen
Ketone —> aldol addition —> aldol condensation
NaOH, H2O
Michael Reaction
1,4 addition
Robinson Annulation
2 Michael additions ( intramolecular)
Malonic Ester Synthesis reagents
NaOEt
R-X
H3O+
Heat
Malonic Ester Synthesis
( malonic ester—> carboxylic acid)
Hoffman Rearrangement Reactants
NaOH, Br2
Heat
Hoffman Rearrangement
amide —> free amine and CO2
Mannich Reaction
RCOR, Formaldehyde, 2ndary amine —> RCOCH2CH2-NR2
Mannich Reaction conditions
HCl, EtOH, 60 C, 2h
Reductive Amination
Imine —> secondary amine
Reductive Amination
NaBH3CN, pH= 3-4
Hoffman Elimination
Least substituted Alkene
Removal of thioalkyl protecting group
Raney Ni, EtOH
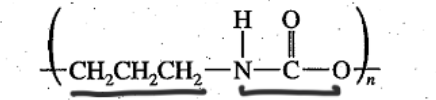
Polyurethane
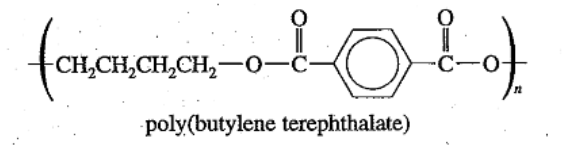
Polyester
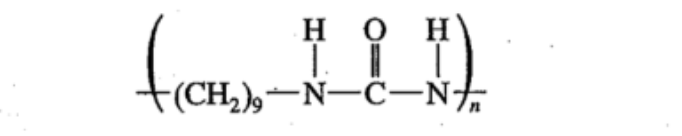
polyurea
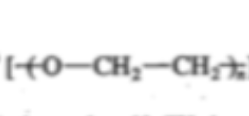
Polyether
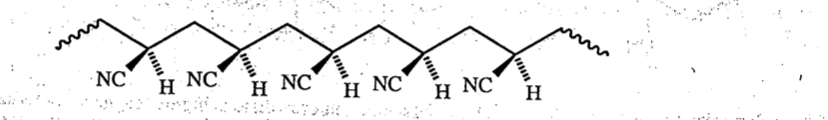
Isotactic
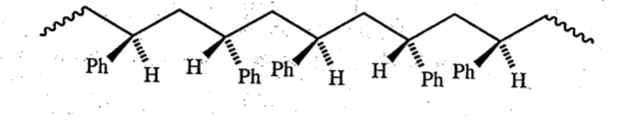
Syndiotactic