CHEM 2302 REAGENTS
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
H2NNH2, KOH
converts aldehydes and ketones into alkanes
NaBH4, Ethanol
ketone to an alcohol
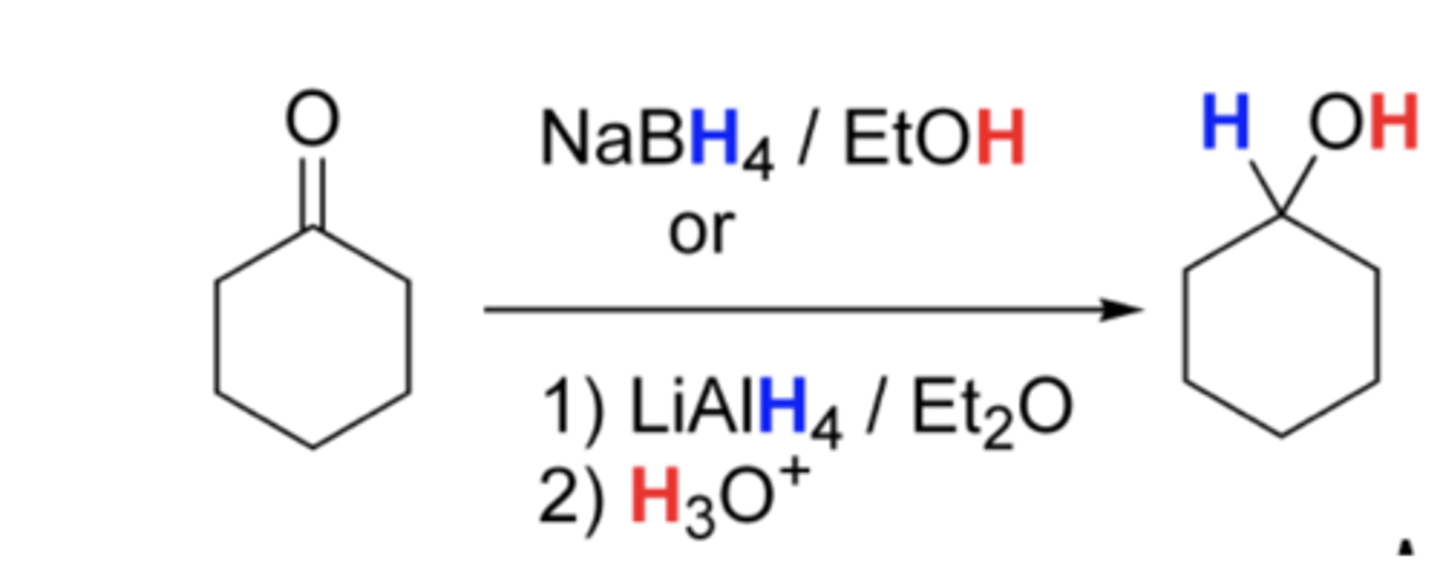
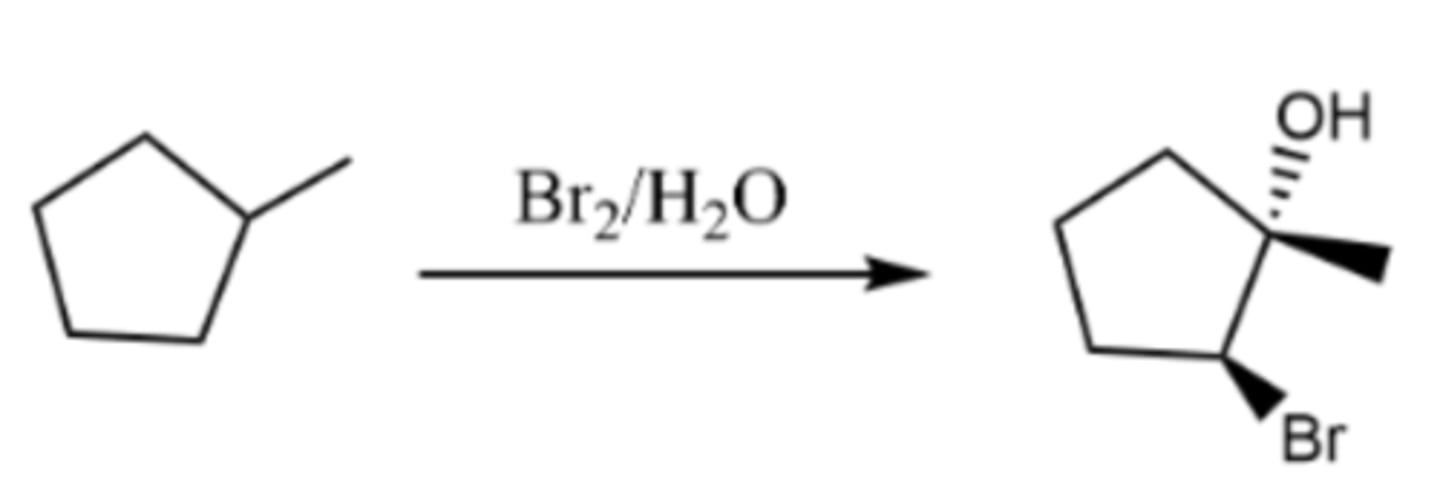
Br2, H2O
halohydrin formation
Friedal-Crafts Acylation
O
II
CH3CH2Cl , AlCl3
remove Cl and add onto benzene
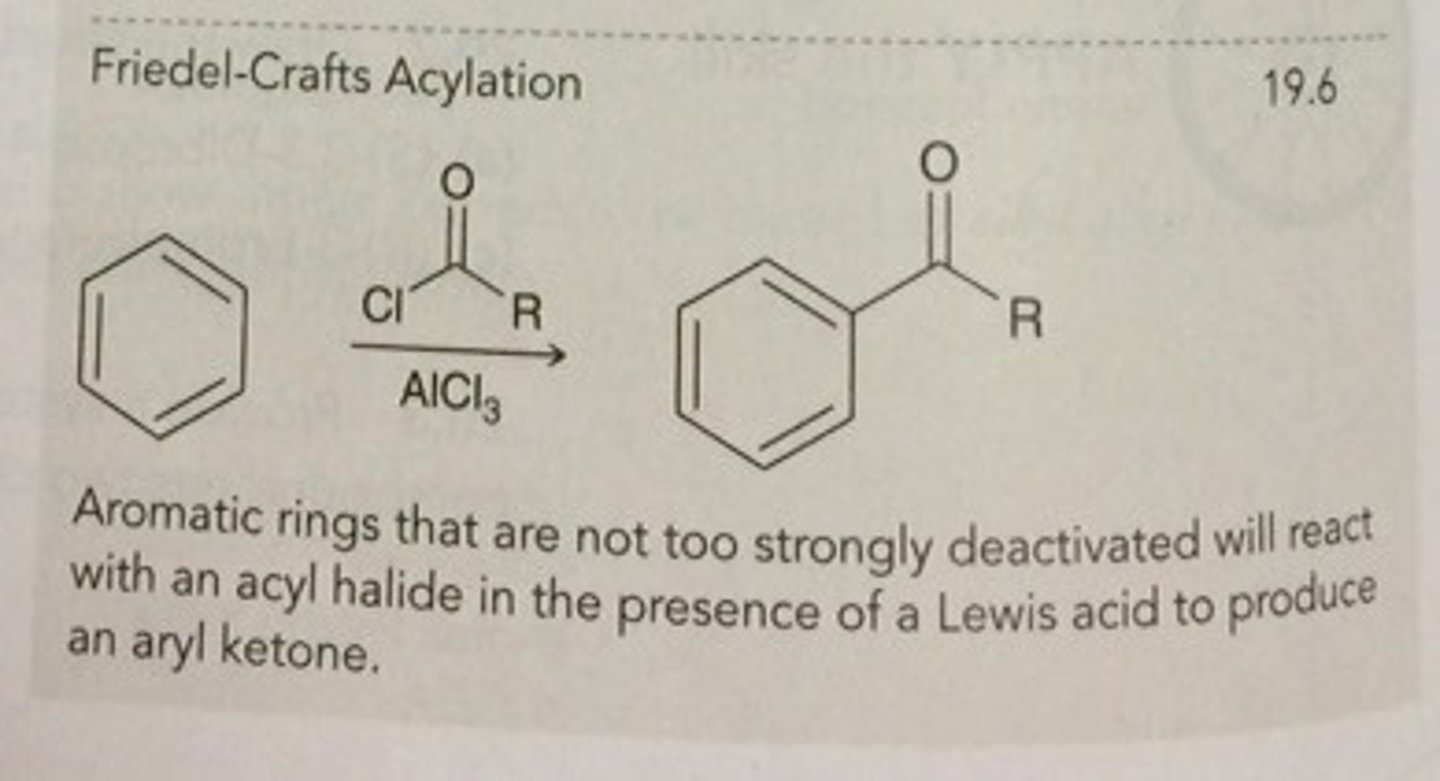
Mg, THF
takes an alkane and forms a grignard reagent (Mg)
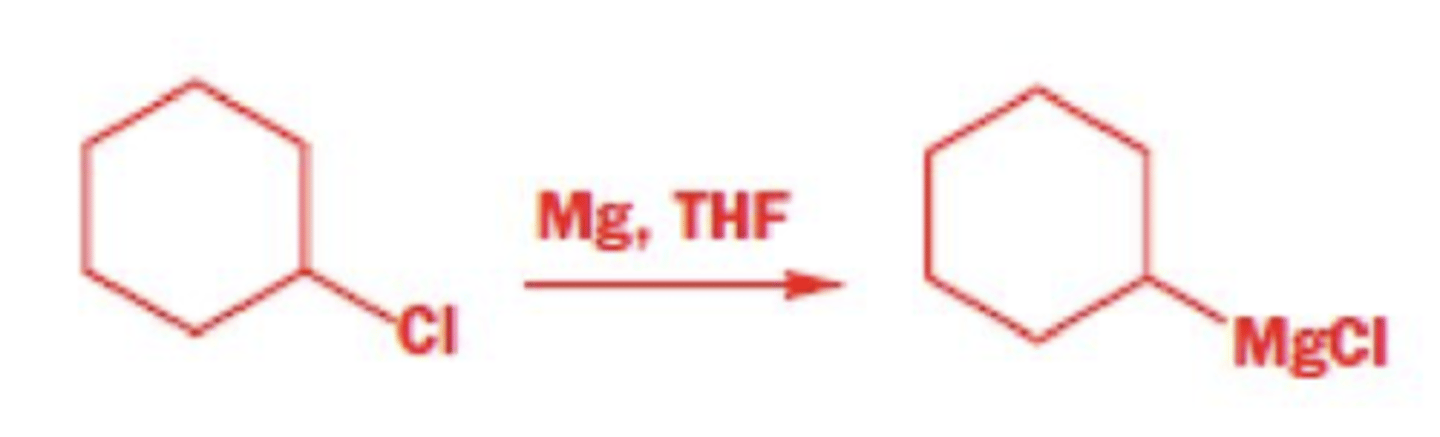
NaH
Williamson ether synthesis
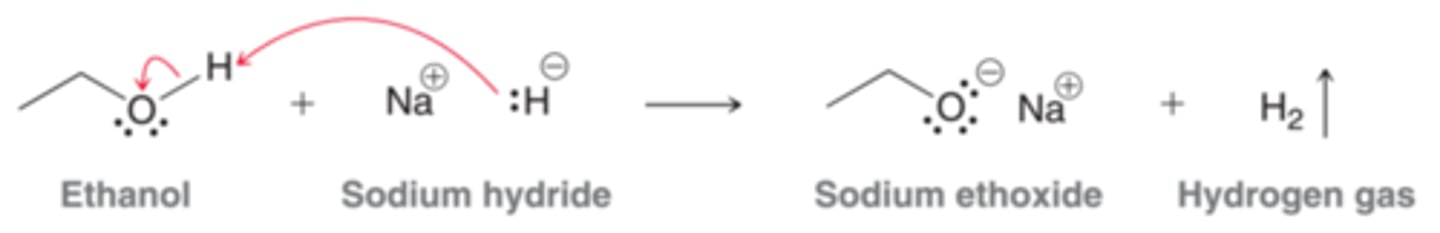
mCPBA
alkene to epoxide
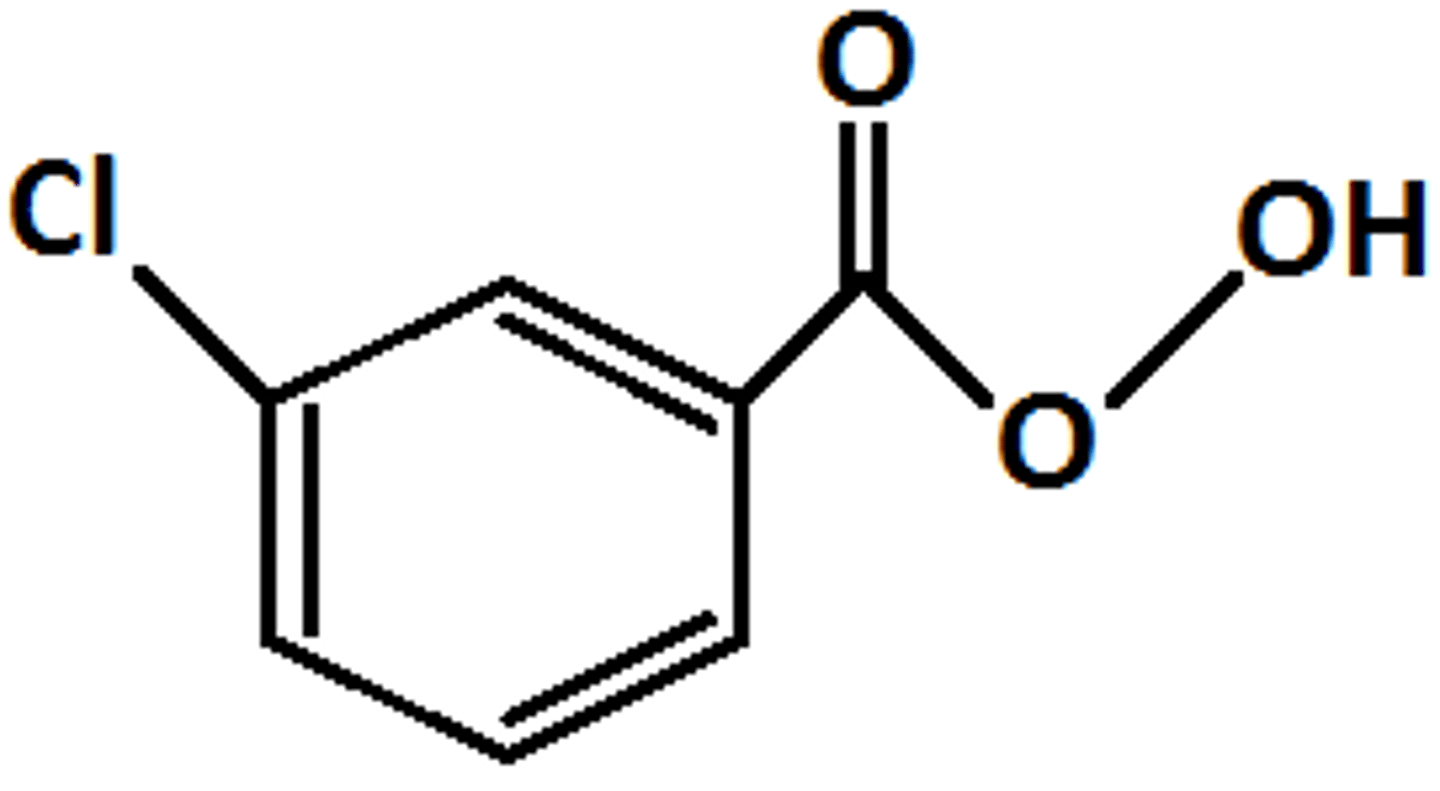
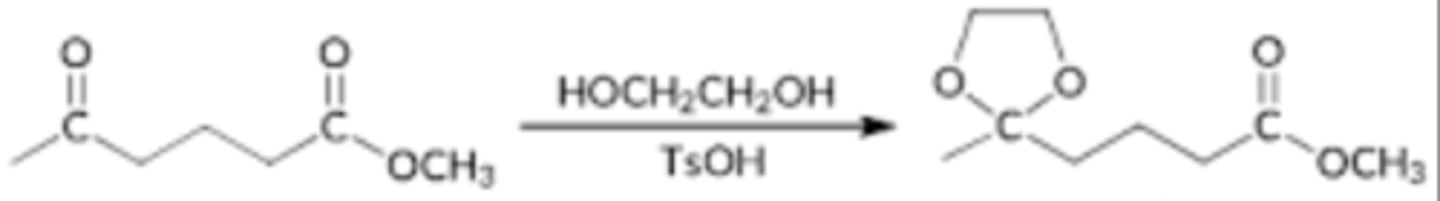
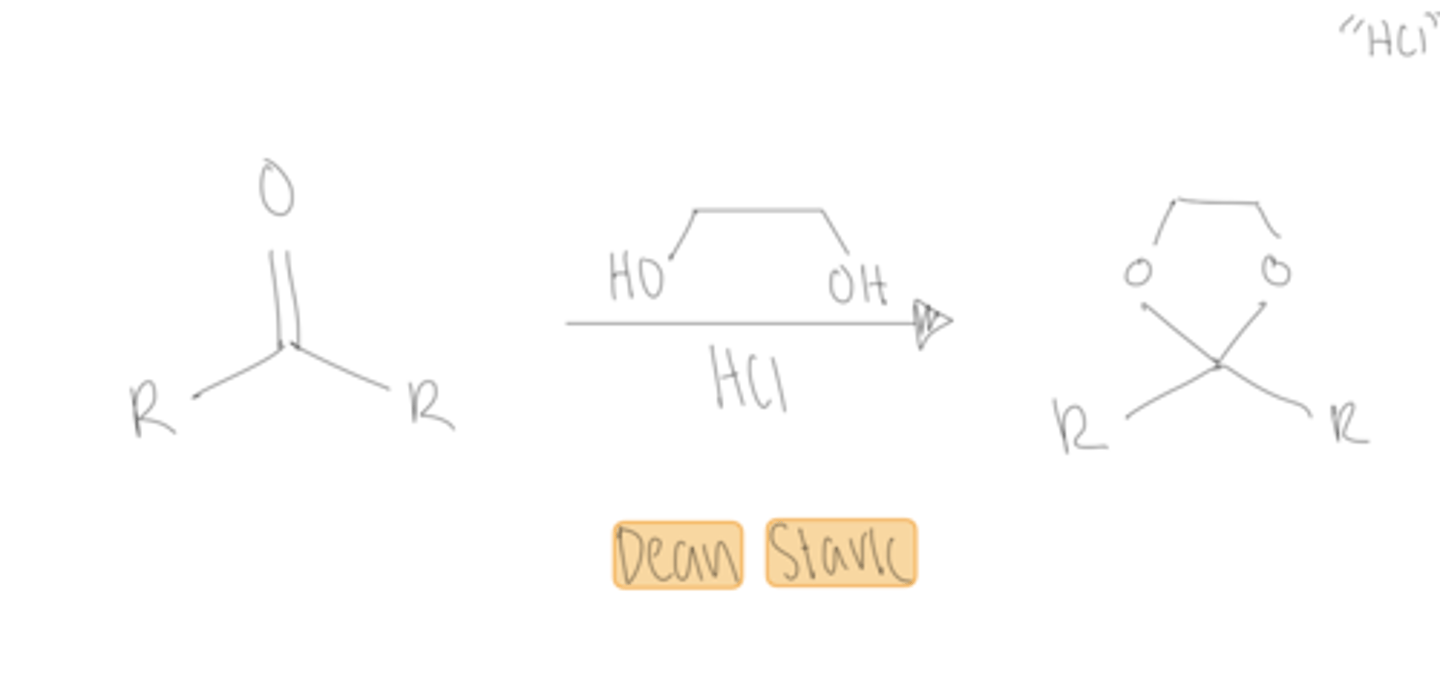
HOCH2CH2OH, TsOH
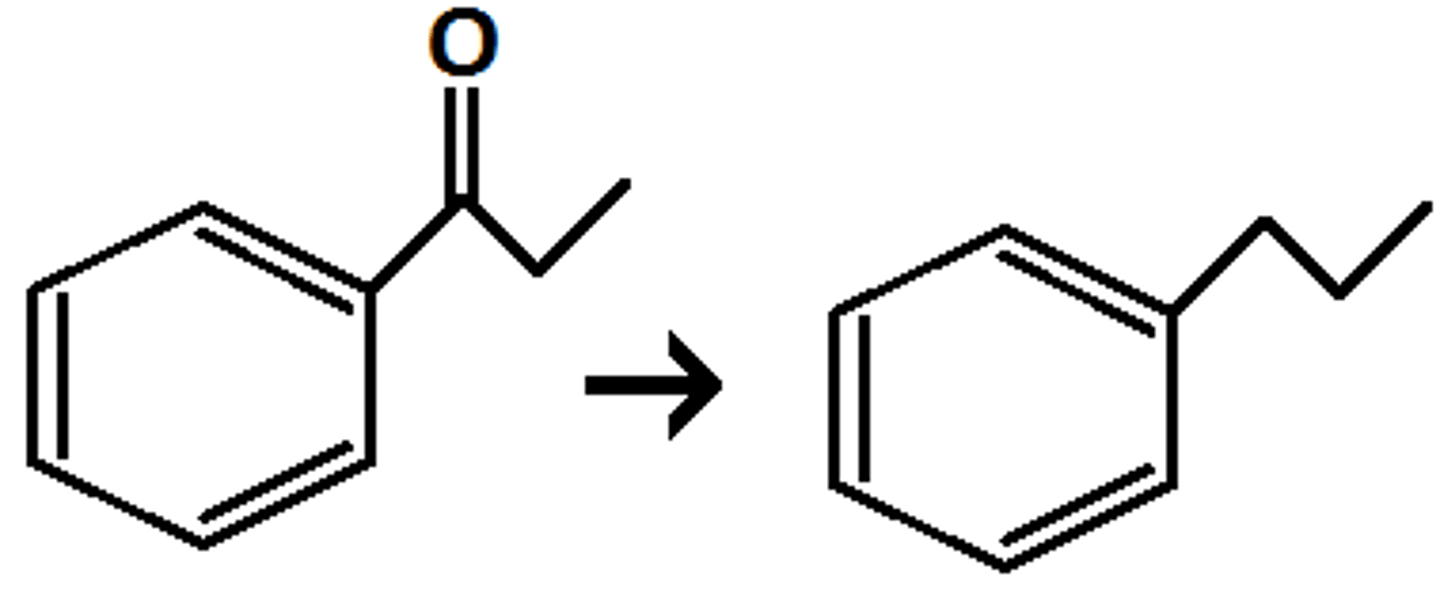
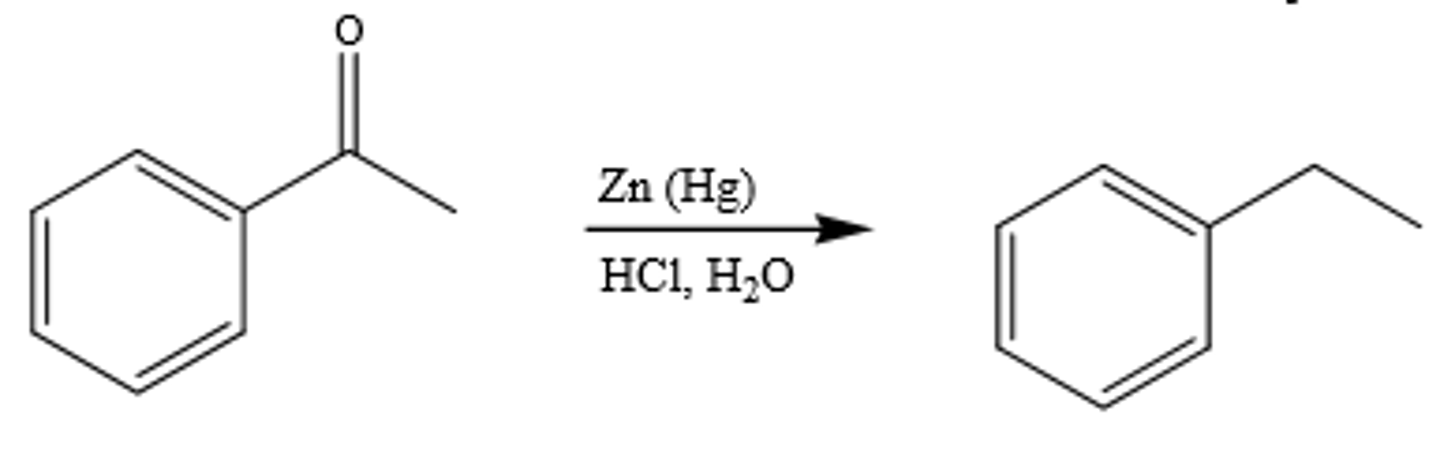
Zn(Hg), HCl
Clemmensen reduction
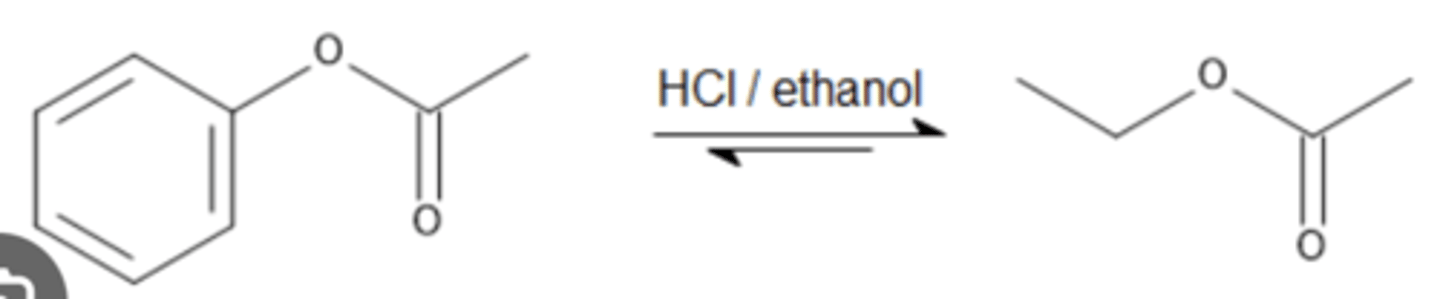
EtOH (excess), HCl
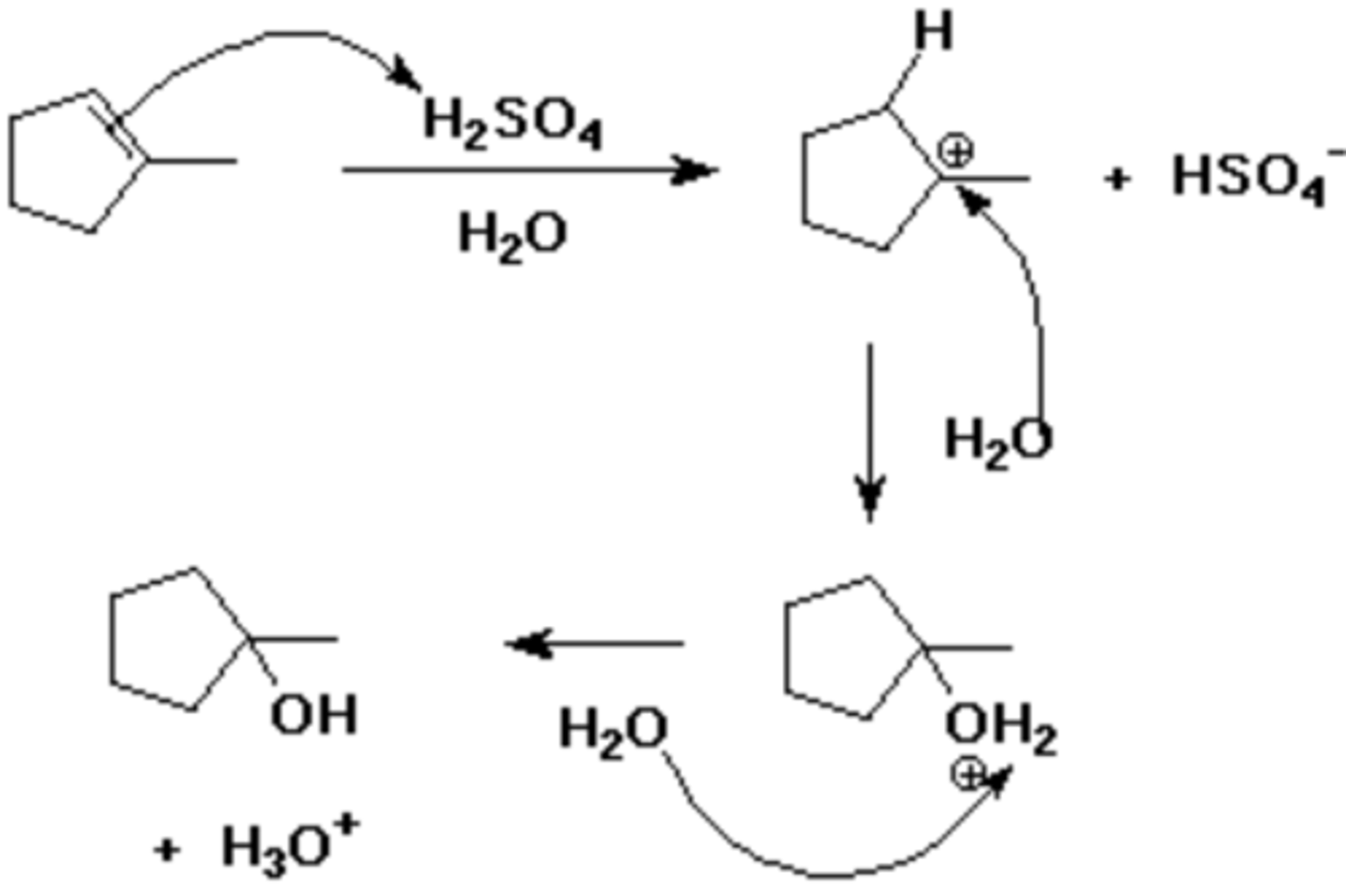
H2O, H2SO4
acid-catalyzed hydration
HCl (excess), heat
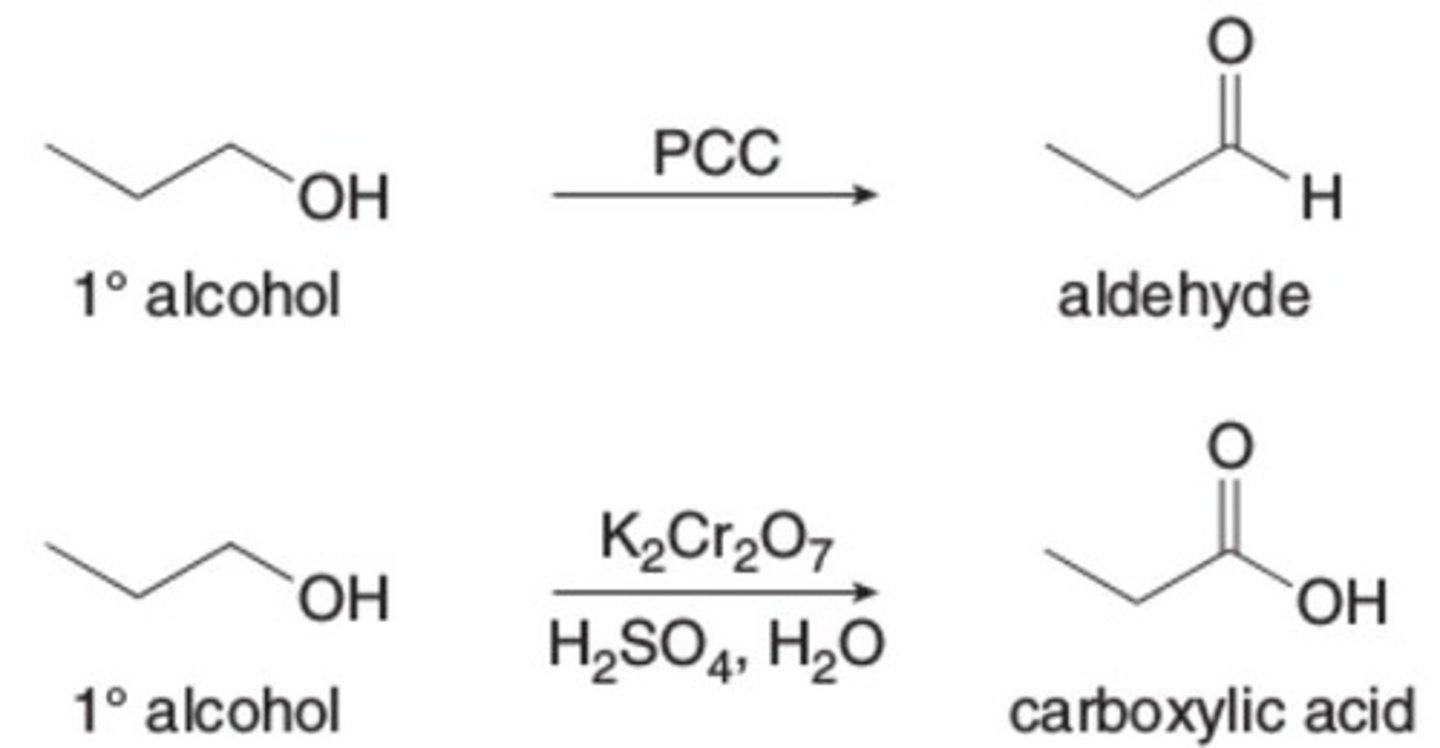
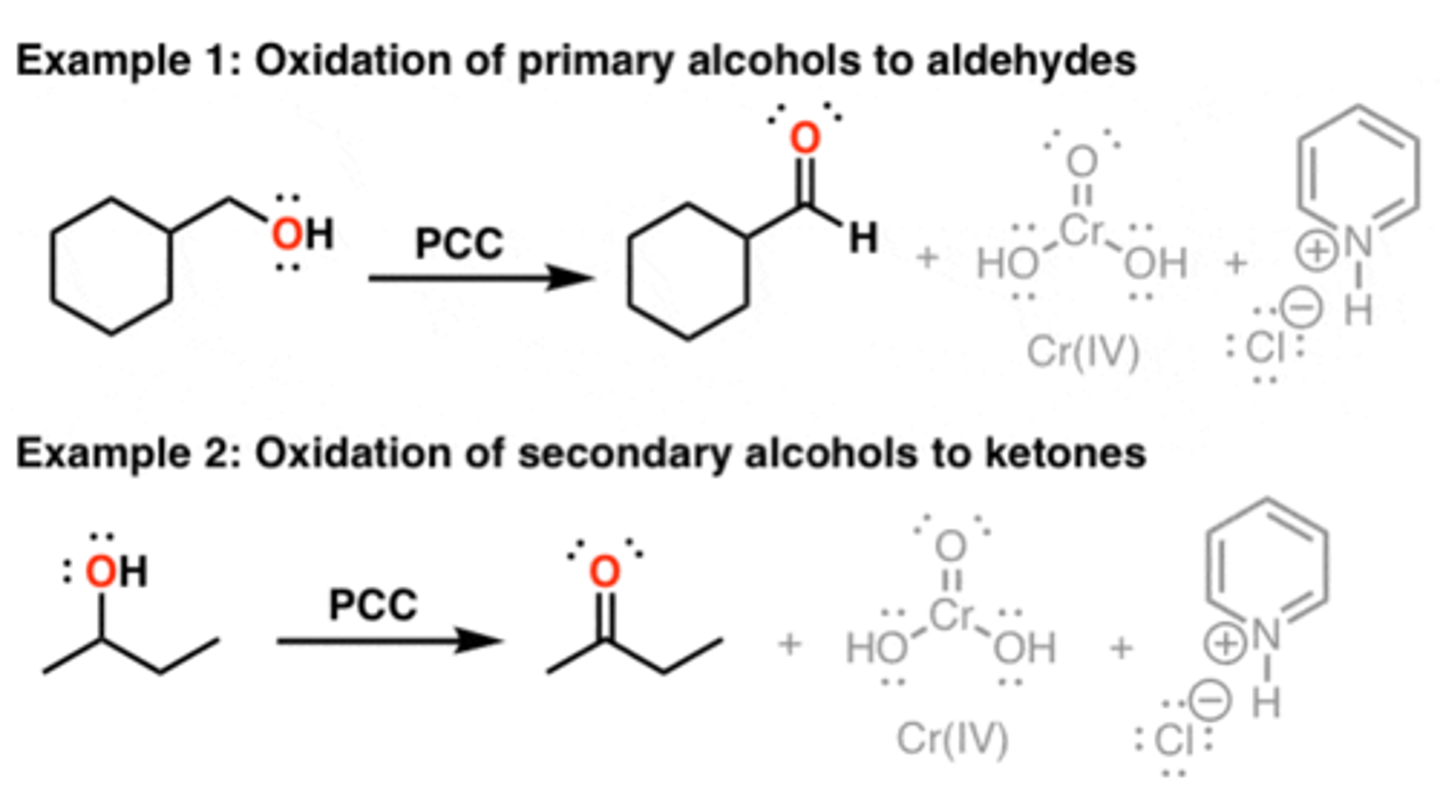
PCC
1) THF
2) H3O+
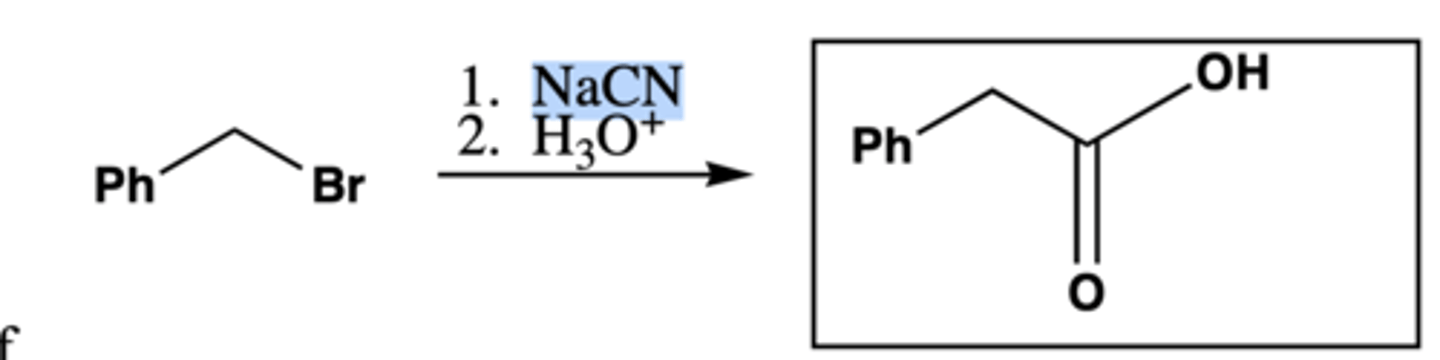
1. NaCN
2. H3O+
Preparation of carboxylic acids: R-CH2-Br
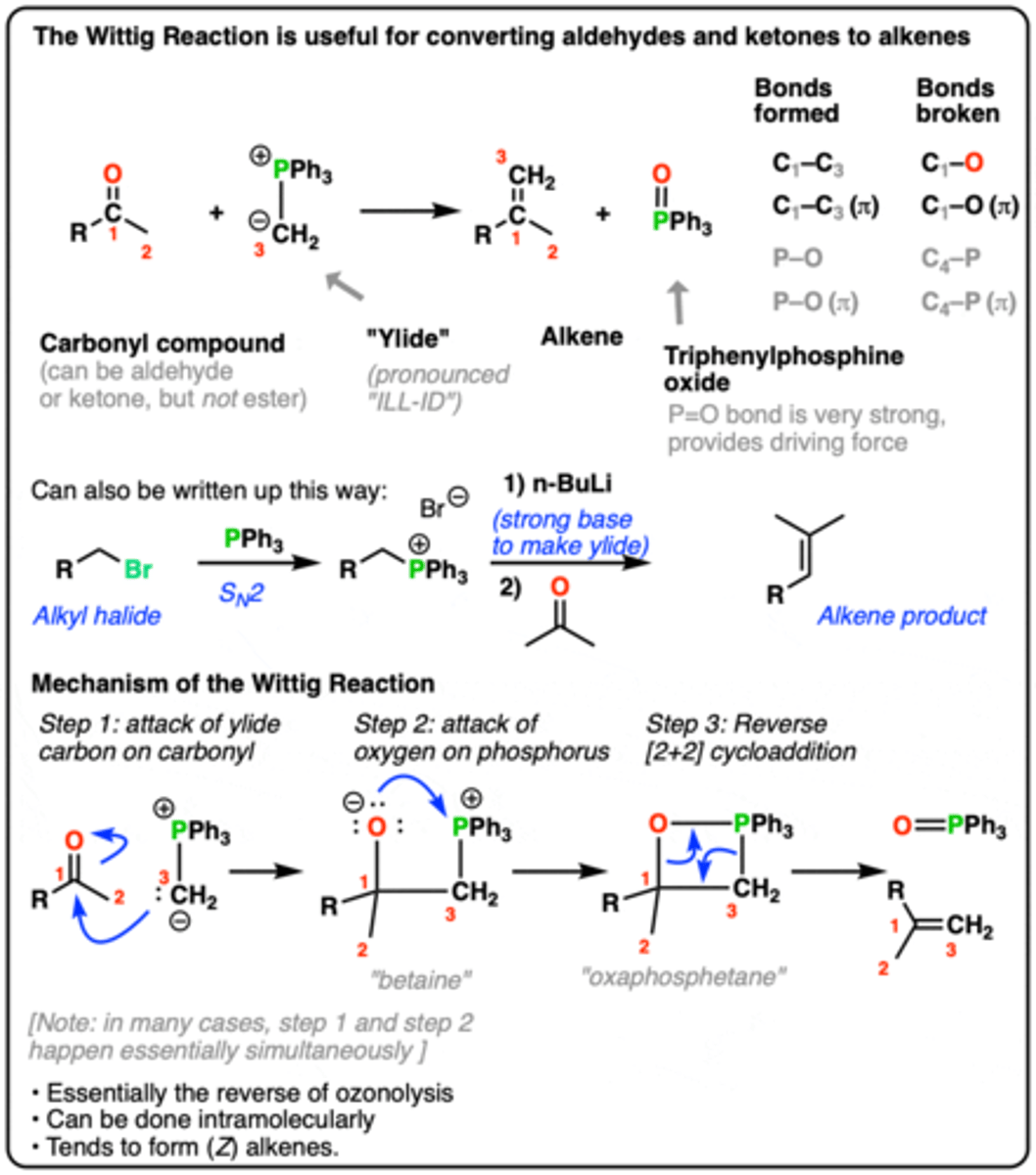
1. [Ph3PMe]+ Br-, nBuLi
2. starting material
ketone to alkene
benzene, dean stark
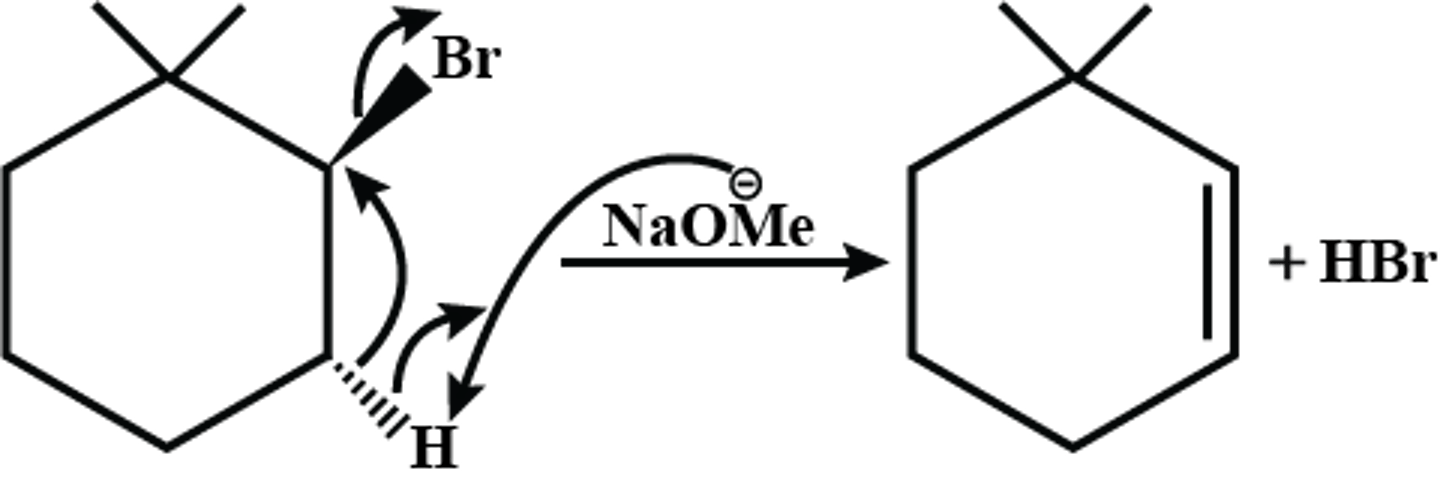
NaOMe
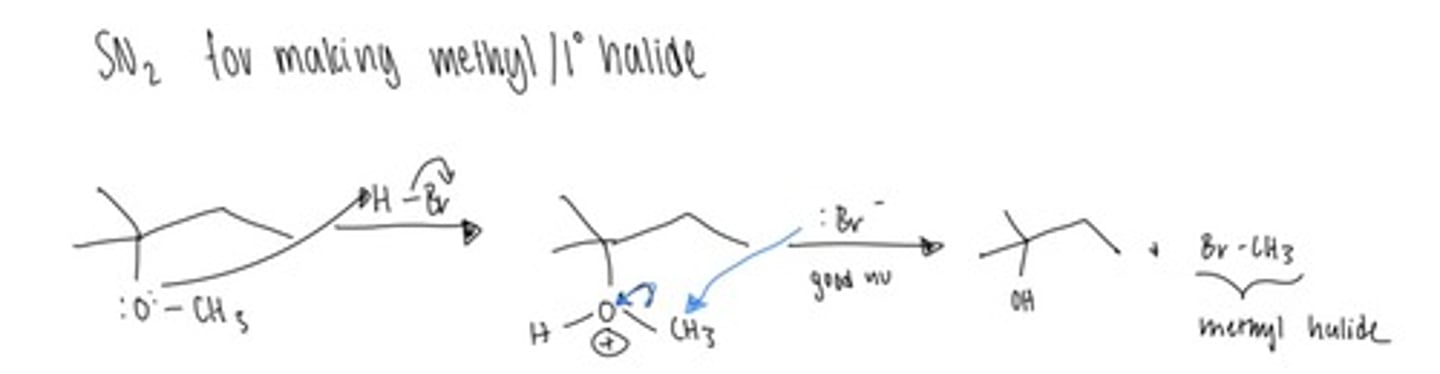
Strong Nucleophile
Strong Base
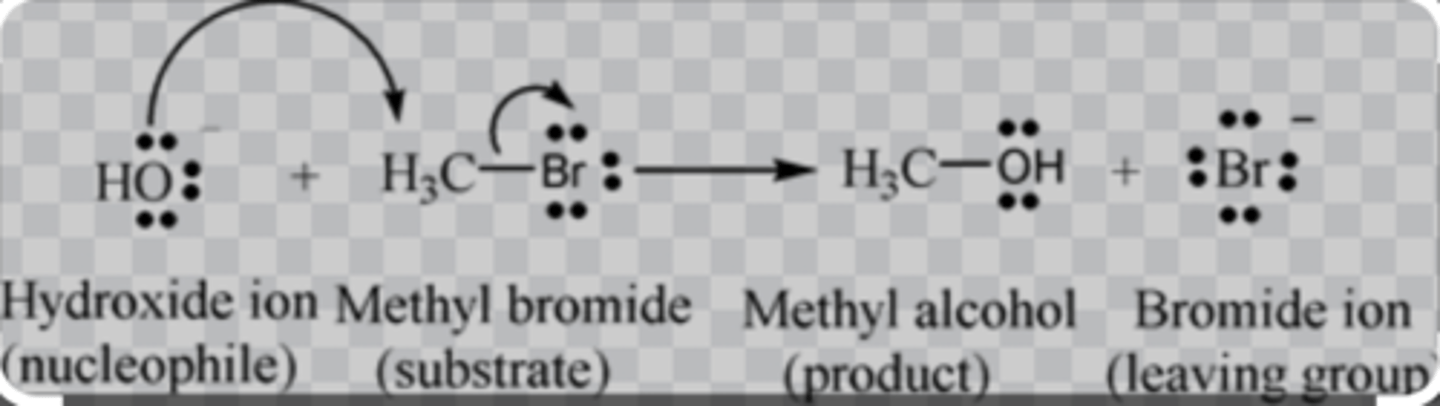
MeBr
1. Li, THF
2. epoxide
3. H3O+
?
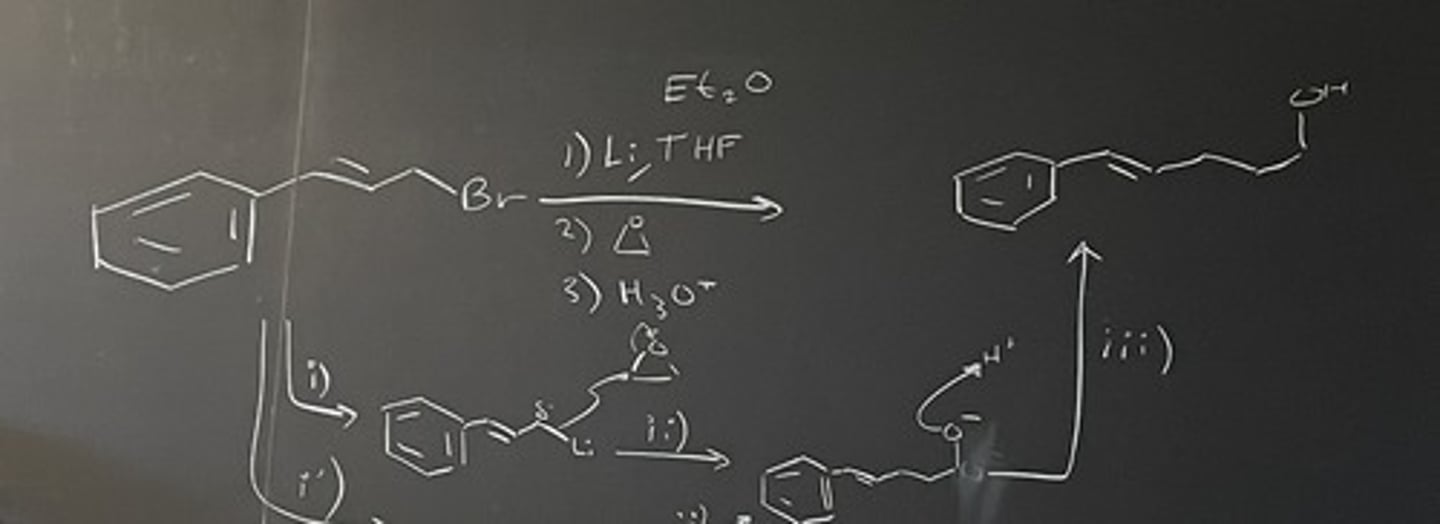
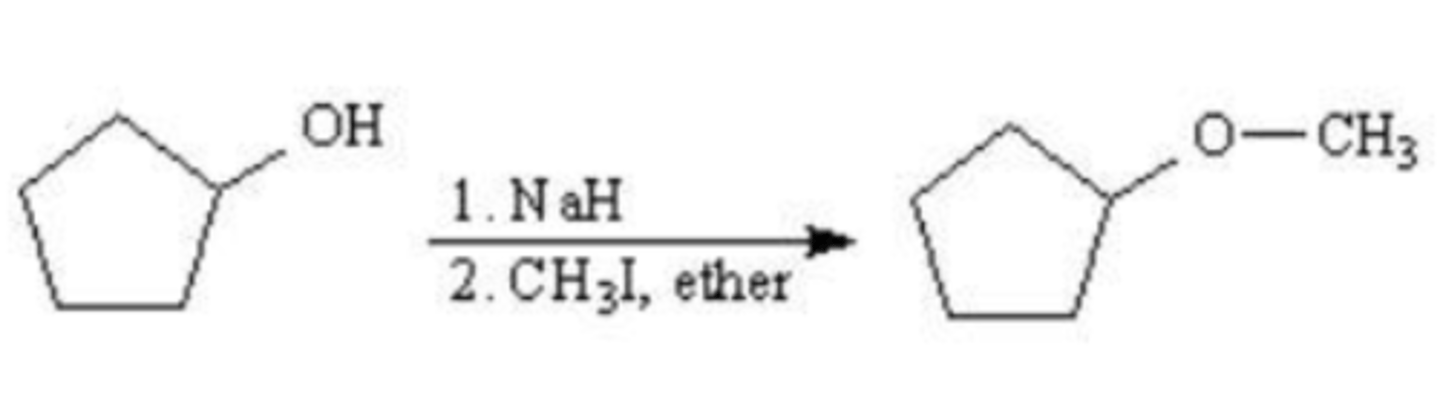
1. NaH
2. CH3I
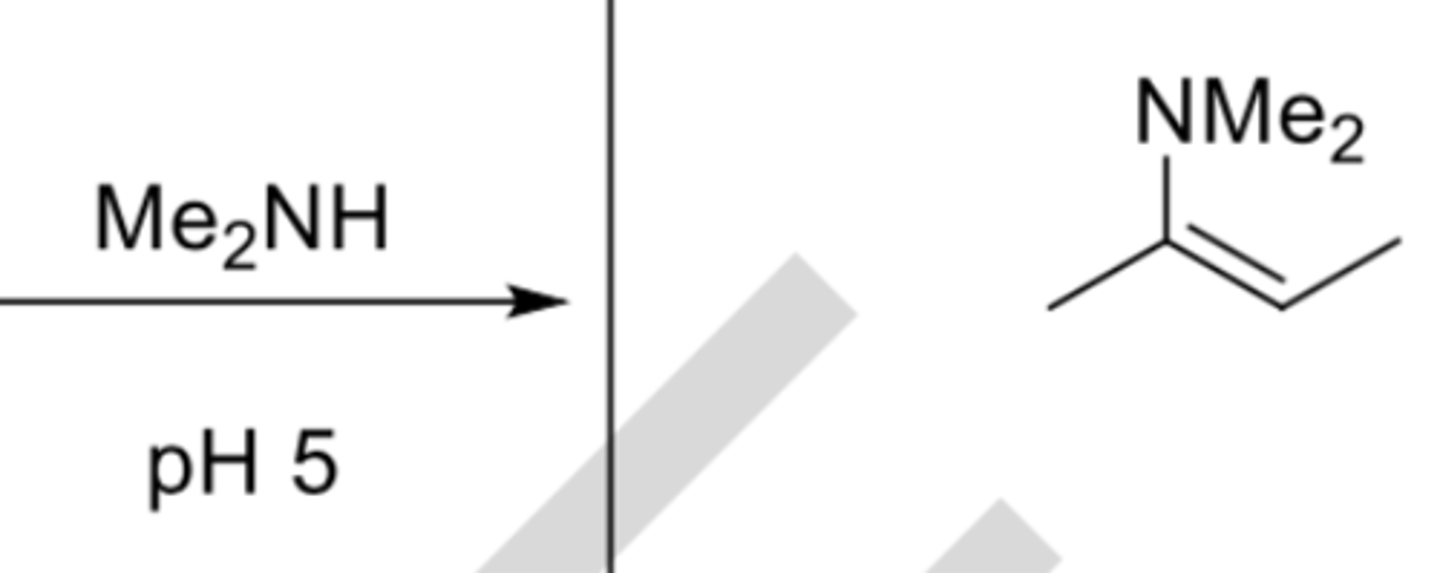
Me2NH
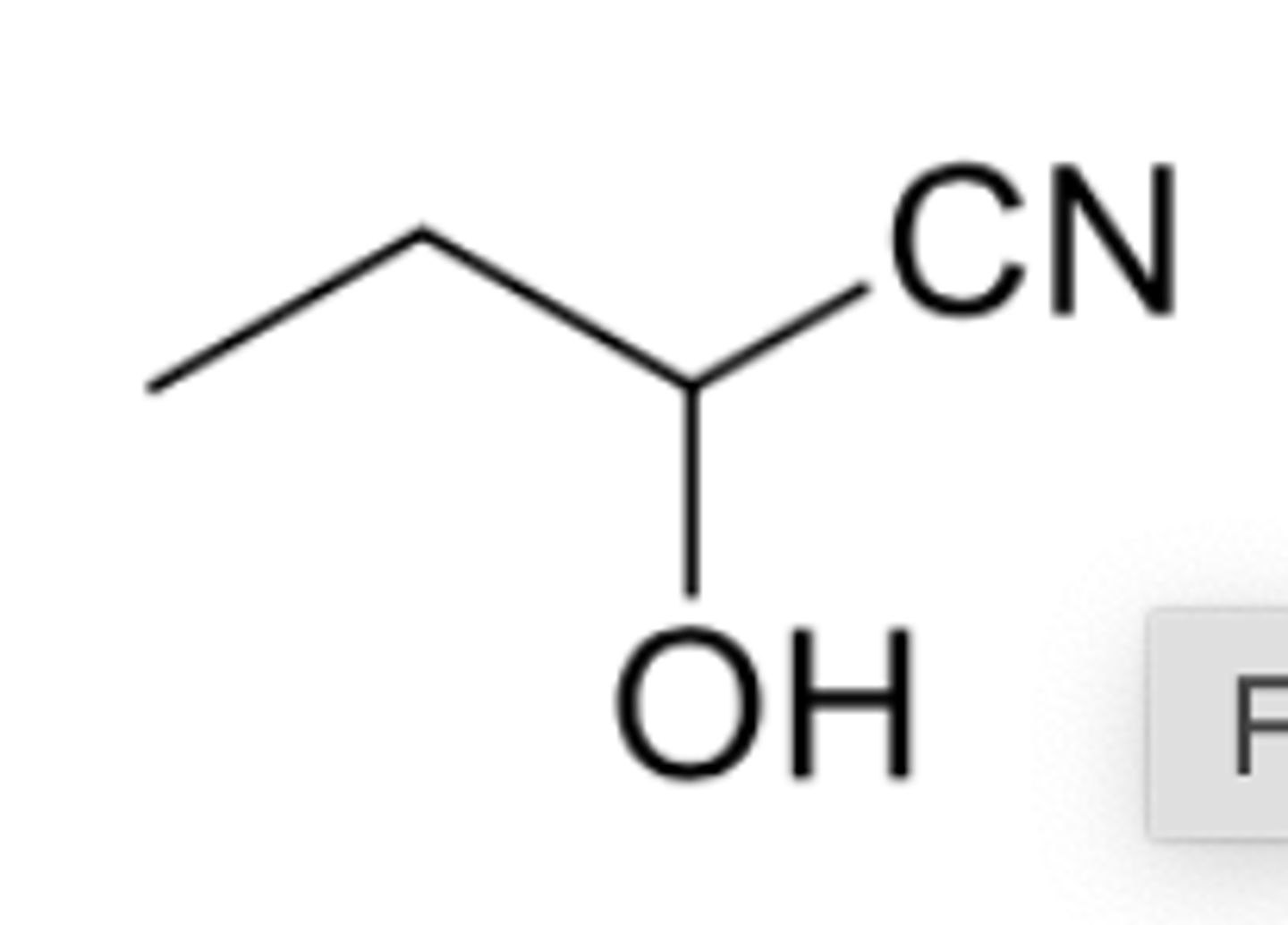
1. PCC 2. HCN, KCN
K2Cr2O7
H2SO4, H2O
makes a carboxylic acid