Chemistry Multiple Choice
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
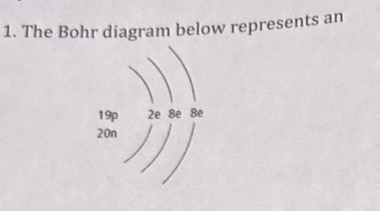
The Bohr diagram represents an
atom of potassium

How many valence electrons are shown in the bohr diagram below?
7
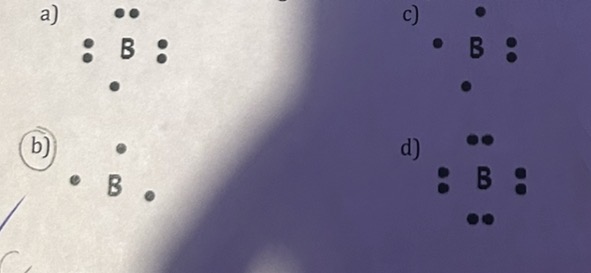
Which of the following Lewis Dot Diagrams represents Boron?
It gains 2 electrons
An element has fulfilled the octet rule. Its electron orbit arrangement is most likely:
b. 2,8,8
Ionic Bonds are generally formed between
A metal and non-metal
Chlorine will form an ionic bond with
Magnesium
Covalent bonds are due to the
sharing of electrons by two atoms
Which of the following is a covalent compound held together by covalent bonds?
Which of the following polyatomic ions have names that end in ate?
i.CLO3-
ii. OH-
iii. PO4 3-
iv. HCO3 2-
v. SO3 2-
a. ii, iii, and iv
The name of the compound CaO is
Select the corresponding name
Fe(Clo3)3 - iron(III) Chlorate
What is the formula for rubidium nitride?
Rb3N
What is the name of the compound of the KMnO4
Potassium Permanganate
The name for PtI2
Platinum (II) Iodide
Which of the coeficcients balances the chemical equation below?
“Use a paper to know how to balance”
2,3,1,3
Which skeleton equation accurately describes the reaction below?
Iron (III) oxide is exposed to carbon Monoxide gas resulting in the formation of solid iron and carbon monxide.
Fe2O3 + CO > Fe + CO2
What type of reaction is shown below?
___Fe + ___H2SO4 > ___Fe2(SO4)3 + ___H2
Single Displacement
Which of the following is an acid?
H2SO4
What are the products of a simple simple neutralization reaction?
Water and Salt