Lecture 30 - Radiation Biology and the Hallmarks of Cancer
1/83
Earn XP
Description and Tags
ONCOL 335 - Radiobiology. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
84 Terms
What were the six original hallmarks of cancer?
resisting cell death
sustaining proliferative signalling
evading growth supression
activating invasion and metastasis
enabling replicative immortality
inducing angiogenesis
what were the four expanded hallmarks?
deregulating cellular energetics
avoiding immune destruction
tumor promoting inflammation
genome instability and mutation
what do cancer cells require to form a macroscopic tumor?
the ability to replicate unlimitedly, uninhibited by normal space restrictions
what limits the amount of cell divisions in normal cells
the Hayflick limit
what happens after the Hayflick limit is reached?
normal cells either face senscence or will undergo cell death
how do cancer cells break the Hayflick limit to allow for immortality?
Cancer cells often will first down regulate P53 to prevent the cancer cell from dying, and then they will activate telomerase, an enzyme that extends telomeres, thus circumventing the normal limits on cell division.
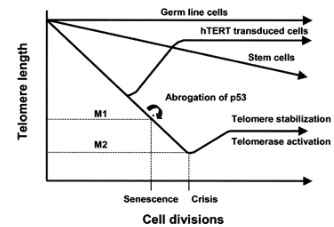
what normal cells have an excess of telomerase
germ cells
so cancer cells essentially will mimic germ cells
what was Theodore Puck’s feeder cell experiment
Theodore Puck's feeder cell experiment demonstrated that normal human cells could be cultured indefinitely by using a layer of feeder cells to provide essential growth factors, illustrating the importance of cellular microenvironments in regulating cell behavior.
this showed that cells need growth factors to grow
now we don’t need feeder cells, we just use growth factors
examples of discoveries that were found with the use of clonogenic survival assays
split dose experiments —> sublethal damage repair
dose rate effects
OER
what future cancer research is under the category of enabling replicative immortality
understand the causes of intrinsic radiosensitivies and taking our knowledge of in vitro killing to the clinic
which cell cycle checkpoint was discovered first? G2 or G1
G2
why was G2 checkpoint discovered first
research was done on HeLa cells, who had G1 inhibited due to HPV inhibiting p53 cell cycle arrest
G1, G2, and G2/M checkpoints are all activated by …
DNA damage
M checkpoint is activated by ….
chromosome misalignment
what gene is required to inhibit or check DNA after IR
ATM
without ATM, the cell can keep proliferating and accumulate additional DNA damage, leading to genomic instability.
what future cancer research is under the category of evading growth supression
research on ATM inhibitors and other drugs that abrograte cell cycle checkpoints
what is the most common type of cell death observed after irradiation
mitotic catastrophe
what caused the discovery that p53 can induce apoptosis
the discovery that radiation to cells increased p53 levels
describe the P53 independent form of apoptosis
IR causes the rapid hydrolysis of sphingomyelin to produce ceramide which activates apoptosis
what other form of cell death can radiation induce
autophagy
what future cancer research is under the category of resisting cell death
can we modeulate p53 response: make drugs that force a mutated p53 to recreate a wildtype p53 response
what happens to chromosomes that have damage by radiation
abnormal chromosomal rearrangements (genome instability)
the study of radiation induce chromosome aberrations led to identifying genes involved in
the DNA damage response and DNA repair
What family are RAD51 and RAD50 genes in
RAD sensitivity genes involved in HR
What family is XRCC1 part of
X-ray repair cross complementing proteins
that play a crucial role in single-strand break repair and cellular responses to DNA damage.
when does the choice between NHEJ and HR occur?
if the DSB gets it’s ends resected
end resection = HR
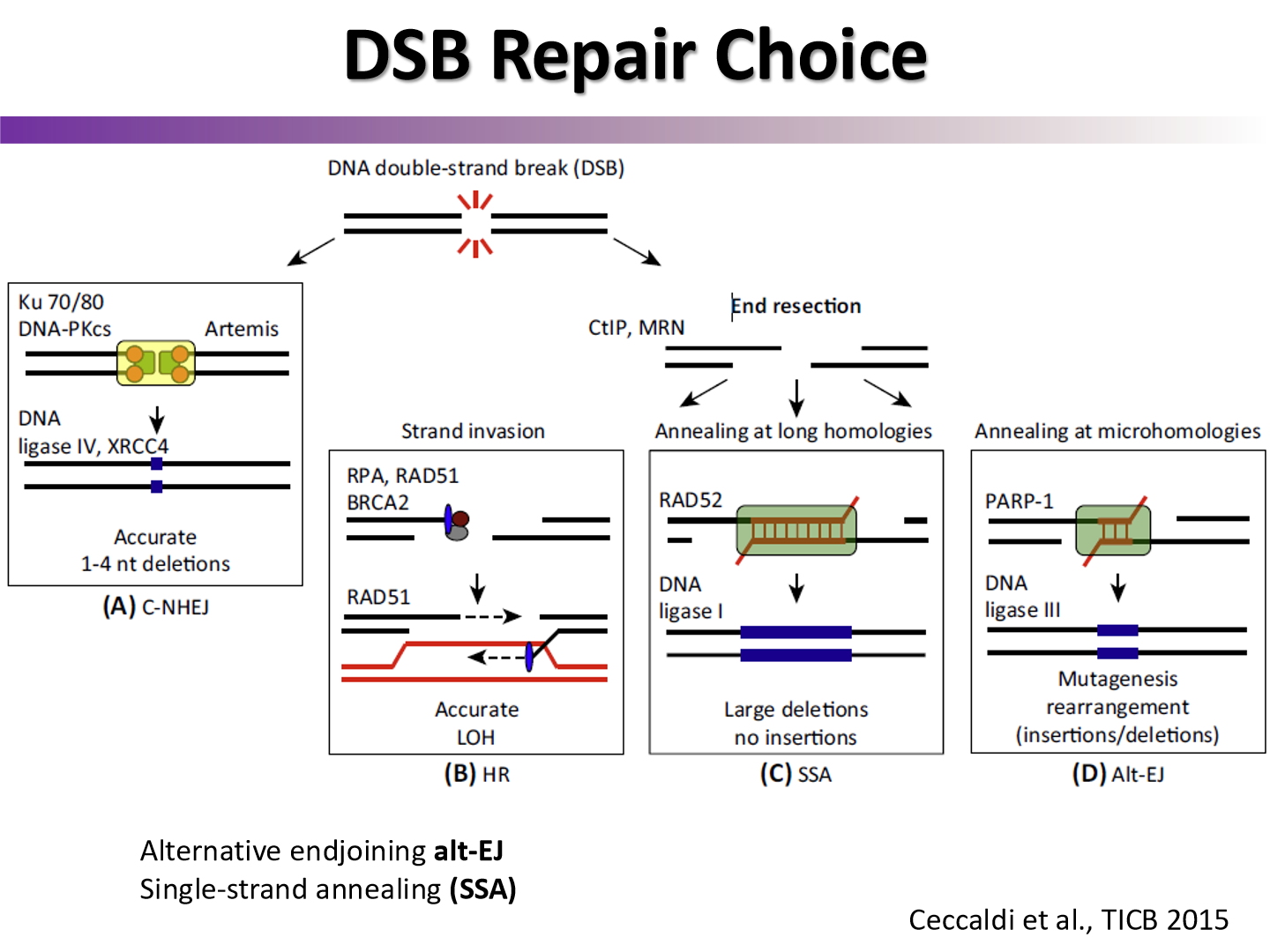
which DNA repair pathway is faster? which one is more faithful?
NHEJ is faster
HR is more faithful due to strand template
what gene was found to control both HR and NHEJ
ATM
who discovered mutations as a result of radiation of cells, and developed the non-threshold linear model for cancer?
Hermann Joseph Muller
How do PARP inhibitors work for BRCA mutant cancers
PARP is inhibited so DNA damage cannot be repaired in cancer cells
PARP is one of the first enzymes that binds to DSB, is thought to recruit other repair enzymes
Acts even before ATM does
what two types of cancers are BRCA mutants
Breast and ovarian cancer
what is synthetic lethality?
The phenomenon where the simultaneous loss or disruption of two or more genes (or genes and small molecules) leads to cell death, while the loss or disruption of any single gene alone does not
what future cancer research is under the category of genome instability and mutation
developing inhibitors of DNA repair enzymes and radiosensitizers
ex: ATM/ATR inhibitors
what was Thomlinson and Gray’s hypoxia experiment?
see how oxygen levels decrease in tumors located away from blood vessels
how far can arteries and veins diffuse oxygen
arteries: more than 70 um
veins: less than 70 um
what two reasons are hypoxic cells problematic for radiotherapy
they are more resistant to radiation as photons rely on indirect damage with ROS
hypoxia can induce factors that create stem cell like properties
stem cells are more radioresistant and metastatic
what two factors levels are raised in tumors after radiotherapy
VEGF
HIF1-a
why do hypoxic tumors reoxygenate after radiotherapy
hypoxic tumors reoxygenate as oxygen consumption rate decreases as a result of cell death of radiosensitive oxygenated tumor cells and increase in perfusion
if you neutralize VEGF before RT, whay happens to tumor shrinkage
Tumor shrinkage may be enhanced, as neutralizing VEGF can improve oxygen delivery and reduce hypoxia, making cancer cells more susceptible to radiation.
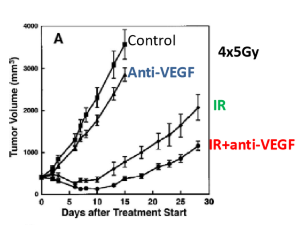
what is a possible explanation as to why HIF1-a increases after radiation treatment
HIF1-a may be stored in stress granules and after irradiation it is released in response to cell stress
ROS stabilize HIF1-a and cause gene transcription/translation
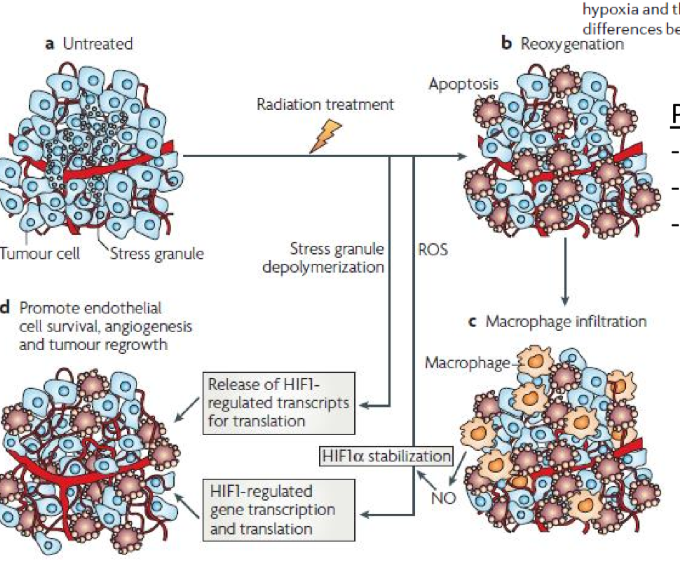
what future cancer research is under the category of inducing angiogenesis
manipulating angiogenesis and tumor vasculature to facilitate perfusion and oxygenation
learning more about role of HIF
What is EMT?
polar epithelial cells lose polarity and connection to eachother to go into lymph and blood vessels to spread
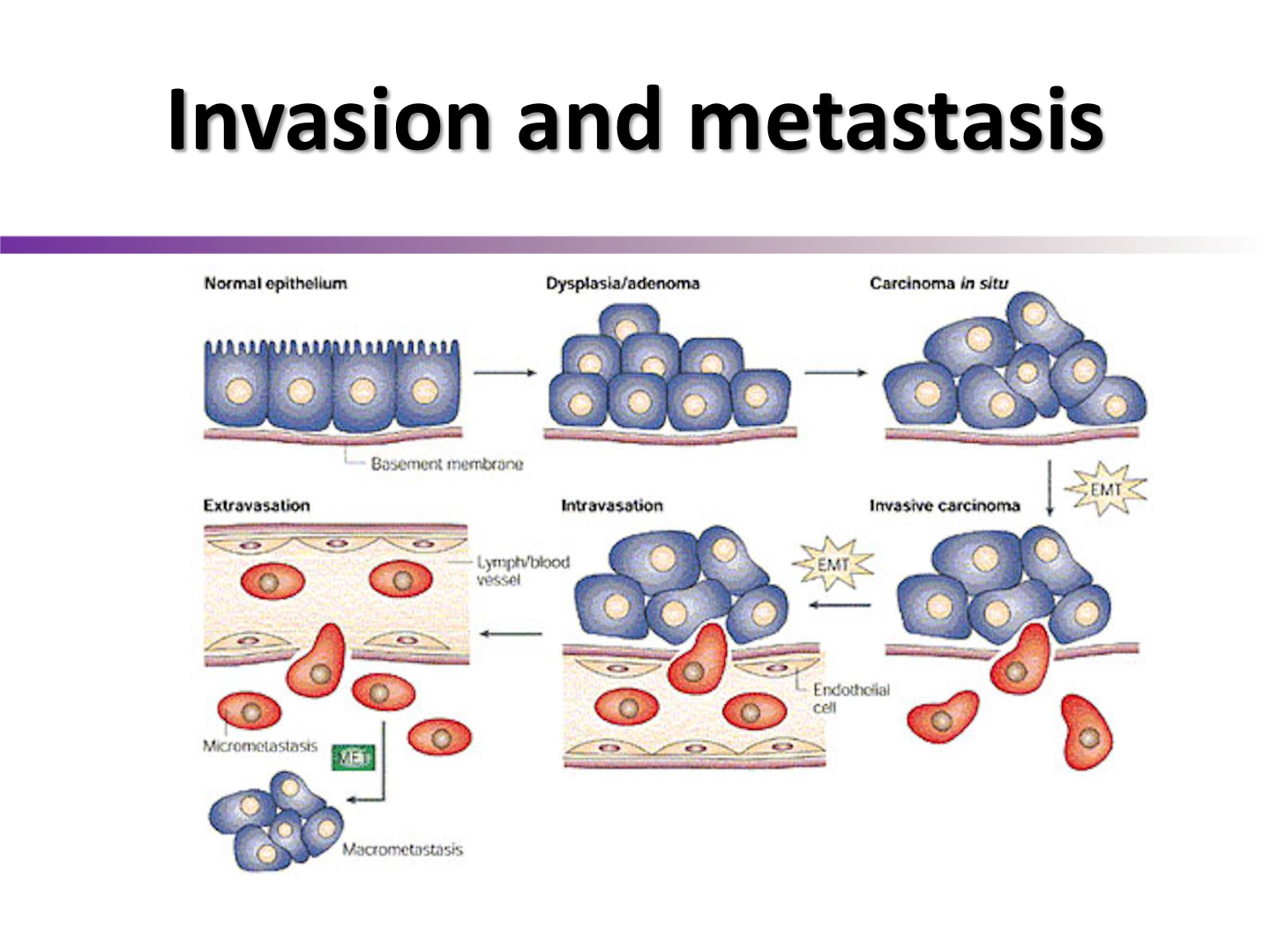
ineffective irradiation of solid tumors can result in ….
enhancement of metastasis
thus we often combine treatments to try to increase outcome
Howcome hypoxia is a strong driver of metastasis
hypoxia upregulates HIF1-a, and is further upregulated with irradiation stress, which may transcribe genes associated with invasion and metastasis
what future cancer research is under the category of activating invasion and metastasis
learning more about IR influencing cell plasticity
creating inhibitors of tumor cell invasion to use as adjuvants to radiotherapy
what is inflammation
the complex biological response of body tissues to harmful stimuli such as pathogens and irritants
what three substances are involved in inflammation
immune cells
blood vessels
molecular mediators
what is the purpose of inflammation
to eliminate the initial cause of cell injury, clear out necrotic cells, and tissues damaged from the injury to initiate tissue repair
how does the inflammatory response contribute to tumorigenesis
The inflammatory response can contribute to tumorigenesis by creating an environment that promotes cell proliferation, survival, and the accumulation of mutations, ultimately leading to cancer development.
when macrophages are irradiated, what two factors did they release
TNF (tumor necrosis factor)
Free radical nitric oxide
has significant consequences for vascular function, inflammation, and cell survival
what is the radiation-induced bystander effect (RIBE)
Cells that are not irradiated but are affected by “stress signal factors” released from irradiated cells. These cells, as well as directly irradiated ones, express DNA damage-related proteins and display excess DNA damage, chromosome aberrations, mutations, and malignant transformation.
What is pyroptosis
Pyroptosis is a highly inflammatory form of programmed cell death that is typically triggered by infection or cellular stress. It involves the formation of pores in the cell membrane, leading to cell lysis and the release of pro-inflammatory cytokines
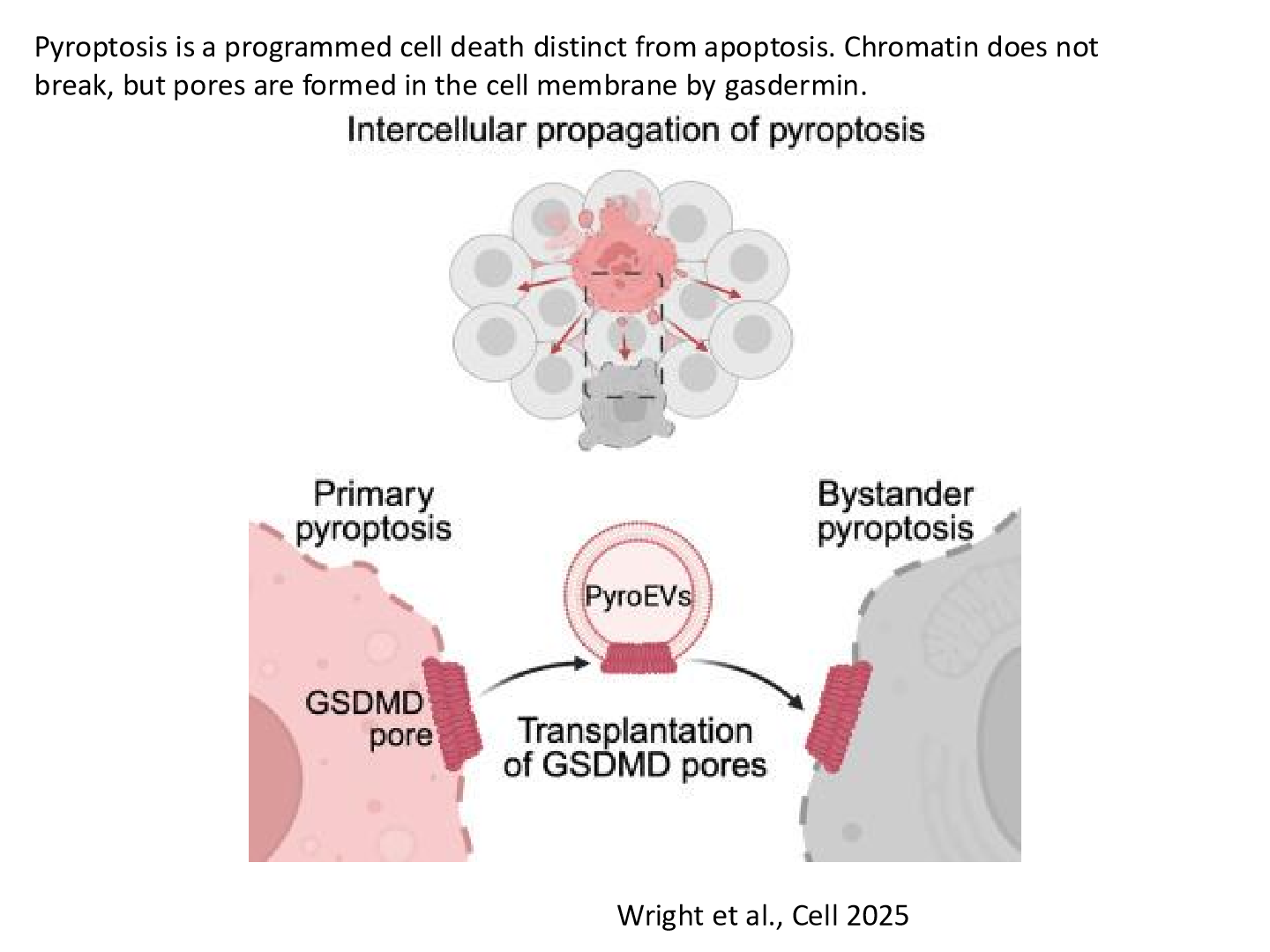
how does pyroptosis affect the RIBE
Pyroptosis enhances the radiation-induced bystander effect (RIBE) by releasing pro-inflammatory cytokines and “stress signal factors” that can impact neighboring non-irradiated cells, leading to increased DNA damage and potential tumorigenesis.
what future cancer research is under the category of tumor promoting inflammation
taking advantage of RIBE effect for radiation therapy
finding ways to modulate inflammatory effects to optimize radiosensitvity of tumors
what factors regulate cellular proliferation and differentiatiation in normal tissues
growth factors
two examples of proteins regulating cell proliferation and survival
EGFR (epidermal growth factor receptors)
Transforming growth factor alpha (TGFa)
what two reasons allow cancer cells to show abnormal growth
they are less dependent on exogenous factors
they are capable of autonomous activation of autocrine and paracrine growth pathways
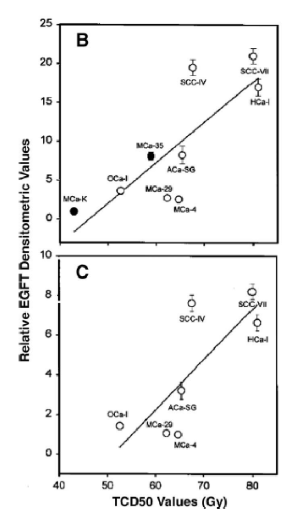
tumors with higher expressions of EGFR are more or less radioresistant
more radioresistant, leading to treatment challenges.
why are tumors with more EGFR more radioresistant
we get increased repopulation of tumors leading to decreased TCP
the more EGFR are the _____ the TCD50
higher
what future cancer research is under the category of sustaining proliferative signalling
create targeted therapies that target proliferative singalling pathways
ex: we use herceptin to target HER2 EGFR pathway
what are the two hallmarks of cancer not included by the 5 R’s of radiobiology?
avoiding immune destruction
deregulating cellular energetics
Immune cells theoretically have the ability to recognize cancer cells based on the cancer associated antigens the cells express on their surface. Why does the immune system fail from catching cancer cells?
tumors have the abiloty to hide their tumor antigens
have secretions that inhibit immune response
what are the most radiosensitive immune cells? what form of cell death do they undergo?
lymphocytes
undergo apoptosis
what type of radiation therapy has been used as a form of immunosupression?
Total body irradiation (TBI)
done before a bone marrow transplant
Based on the radiosensitivity of immune cells at the tumor, it was orginially thiought that irradiation was immunosuppressive. However, later research has found certain doses can lead to the upregulation of tumor associated antigens on cancer cells, resulting in immunogenic cell death. what level of doses cause increased immunogeneicity?
above 10 Gy we seem to get better immune response
what type of radiation therapy may result into making tumor cells more immunogenic?
SBRT (Stereotactic Body Radiation Therapy)
high doses, hypofractionated
what is an abscopal effect
damage to the primary site may release tumor associated antigens into the environment to be picked up by immune cells. this may result in the regression of disease in metastatic sites
combining radiation therapy with _____ can trigger anti-tumor immuniry and lead to regression of the disease
immunotherapy: monoclonal antibodies agaisnt immune checkpoint inhibitors
CTLA-4, PD1
what is the ideal abscopal effect situation
An ideal abscopal effect situation occurs when localized radiation treatment not only reduces the primary tumor but also induces systemic anti-tumor immune responses that lead to the regression of untreated metastases.
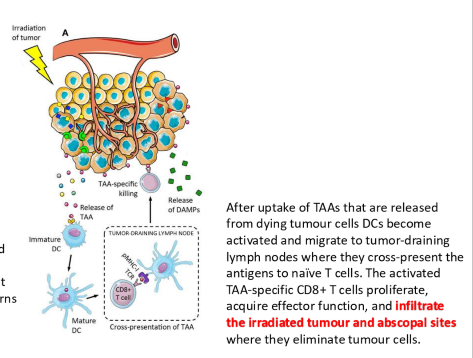
what are four issues that may inhibit an abscopal response
hypoxia may supress immune cells
tumor death may release chemocines to transform immune cells to M2 phenotype
TGF-B signals may transform immune cells to M2 phenotype
tumor death releases ATP, and adenosine can inhibit immune cells
what future cancer research is under the category of avoiding immune destruction
developing immunomodulators to improve radioresponse and anti-tumor immunity
learn more about influence of immune system on TME
does the way a cell die matter for an immune response
yes, some cell death mechanisms lead to better immune response
apoptosis leads to poor immune response
what three ways does the TME differ from a normal cell environment
hypoxia
lower pH
nutrient deprivation
under aerobic conditions, what does metabolism look like
Normal metabolism relies on oxygen for energy production, primarily through oxidative phosphorylation, resulting in efficient ATP generation and low lactate production.
what cycle will aerobic metabolism undergo
Krebs cycle (Citric Acid Cycle)
under anaerobic conditions, what does metabolism look like?
Metabolism shifts to anaerobic glycolysis, resulting in less efficient ATP generation and increased lactate production, often leading to acidosis.
what process will anaerobic metabolism undergo
fermentation: to generate ATP and produce byproducts like ethanol or lactic acid.
what did Otto Warburg win a nobel prize for?
Discovering the Warburg Effect
what is the Warburg Effect?
a phenomenon where cancer cells preferentially produce energy through aerobic glycolysis rather than the more efficient oxidative phosphorylation, even in the presence of ample oxygen. This means they convert glucose to lactate instead of fully breaking it down in the mitochondria.
Three reasons why cancer will choose aerobic glycolysis over OxPhos
It provides intermediates for biosynthesis (nucleotides, amino acids, lipids)
it alows for faster ATP production (though less efficient)
it creates an acidic tumor microenvironment, which may aid in invasion and immune evasion
what role does HIF1 have in aerobic glycolysis?
increases glycolytic metabolism and increase glucose transporter expression
how do we know HIF1 affects aerobic glycolysis
in HIF1 depleted tumors, ATP levels are lower
what future cancer research is under the category of deregulating cellular energetics
developing glycolysis inhibitors to add to RT treatments to potentially improve outcome
learning more how metabolism influences radiation response