Physics II Lesson 6: Spectroscopy Part III
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
CRB Which of the following types of molecules can have their peculiar proton chemical shifts explained by Diamagnetic Anisotropy?
I. Aromatic Compounds
II. Huckel-Shaped Compounds
III. Antiaromatic Compounds
(A) I only
(B) I and III only
(C) II and III only
(D) I, II and III
(B) I and III only
Diamagnetic Anisotropy can be used to explain the peculiar chemical shifts in Aromatic and Antiaromatic compounds.
How does Diamagnetic Anisotropy explain the fact that conjugated compounds are farther downfield than would be expected?
It illustrates that protons outside a ring will have a magnetic field (βin) that is in line with the external magnetic field (β₀). This results in a larger βeff than is normally seen.
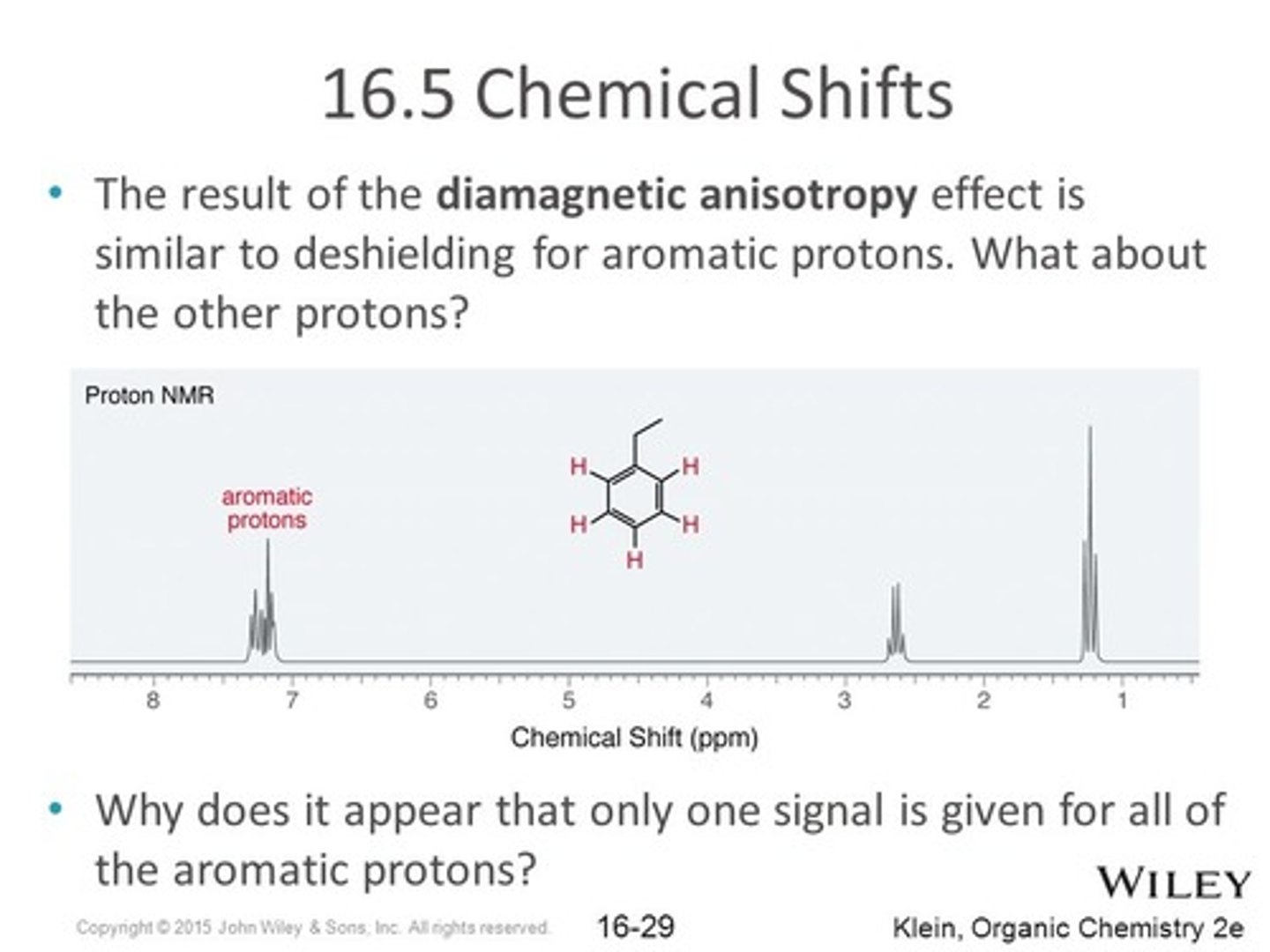
CRB Imagine that one carbon-carbon bond is broken in a Benzene ring, and the product is hydrated to give C6H8. Would this still experience Diamagnetic anisotropy? Why or why not?
This would not experience Diamagnetic Anisotropy, because this compound is both not aromatic and not a ring, the protons will not be aligned in a way to mirror and increase the influence of the external magnetic field.
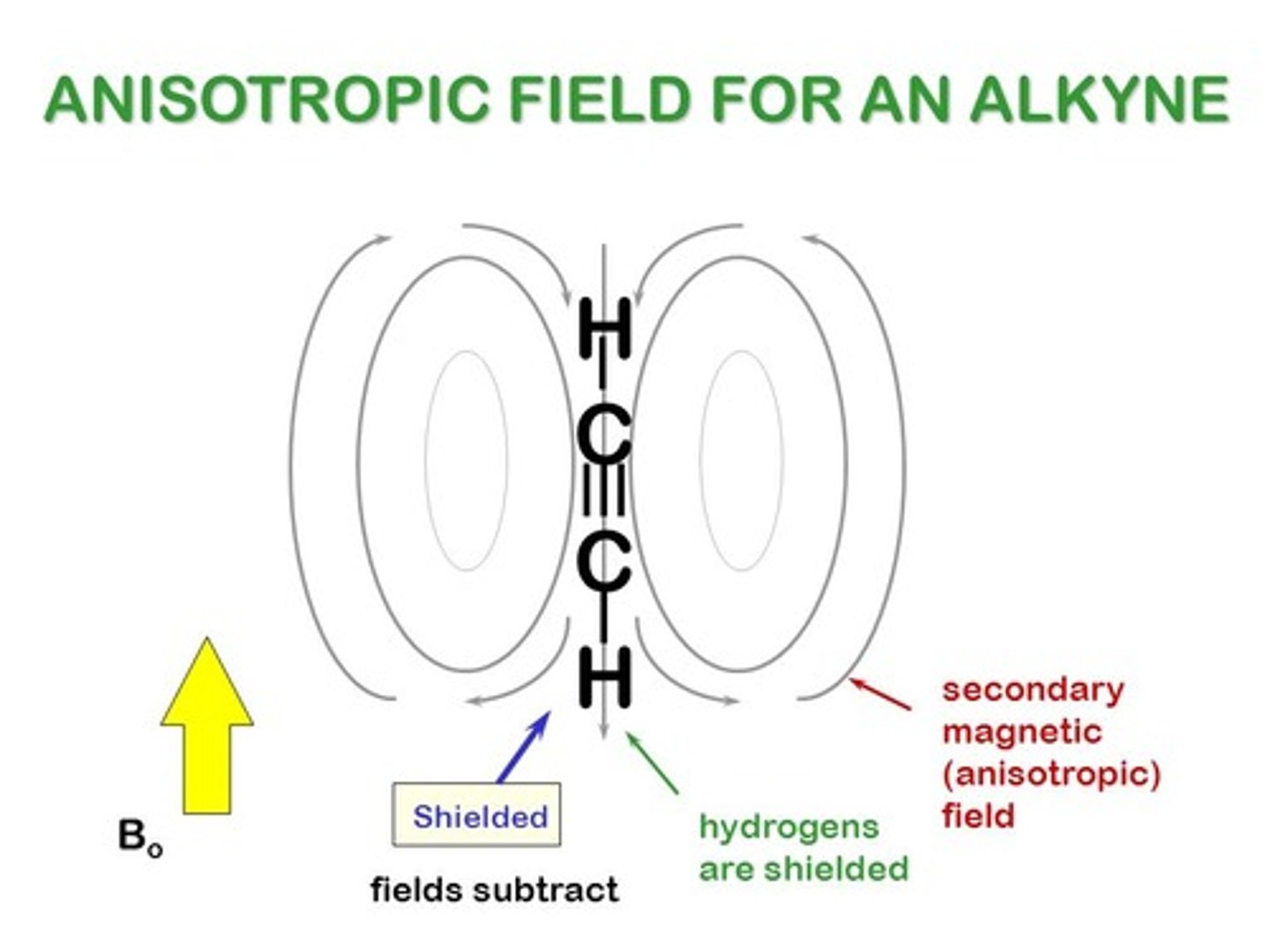
How does Diamagnetic Anisotropy explain the fact that a benzene's hydrogens appear more downfield than an alkyne's hydrogen?
It illustrates that protons of an alkyne will have a magnetic field (βin) that is opposite the external magnetic field (β₀). This results in a lower βeff than is normally seen.
Conversely, the Benzene ring has a magnetic field (βin) that is aligned with the external magnetic field (β₀), creating a higher βeff than is normally seen, causing it to have a larger (downfield) chemical shift.
What information do Integration values tell you?
Integration values are the area under the curve. They tell you how many protons or carbons are represented by that peak.
A given compound has 10 hydrogens. There are 4 peaks with integration values of 57.6, 11.3, 11.4, and 35.6. How many hydrogens are associated with the peak that has an integration value of 57.6?
(A) 3
(B) 4
(C) 5
(D) 6
(C) 5
I would notice that the lowest value is 11.3, which when divided by the total gives you a value close to .1; thus, I would know that this peak corresponds to 1/10th of the total hydrogens (10). Representing a single hydrogen. Because 57.6 is about 5 times larger, it must represent 5 hydrogens.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB What if you are given the integration values of 27.1, 40.1 and 40.9? How many protons correspond to the 40.1 value?
(A) 2
(B) 3
(C) 6
(D) Not enough information
(D) Not enough information
I notice that the total number of Hydrogens is not given, so I can only establish a ratio between the values.
CRB What if you are given the integration values of 27.1, 40.1 and 40.9? What is the likely ratio of protons in each of the three groups?
(A) 1:2:2
(B) 2:3:3
(C) 3:4:4
(D) 27:40:41
(B) 2:3:3
Looking at this, I can round these numbers off to 27, 40.5 and 40.5, and I see that if you divide by a factor of 13.5, you can see a 2:3:3 ratio.
You could also try dividing 40.1 by 27.1, and get about 3/2.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is Splitting and when does it result? Describe what is the cause of Splitting.
Splitting is the splitting of a peak that occurs when you have nonequivalent protons next to each other. It is caused by the idea that the environment of a proton will be different when the neighboring proton is in its β versus α state.
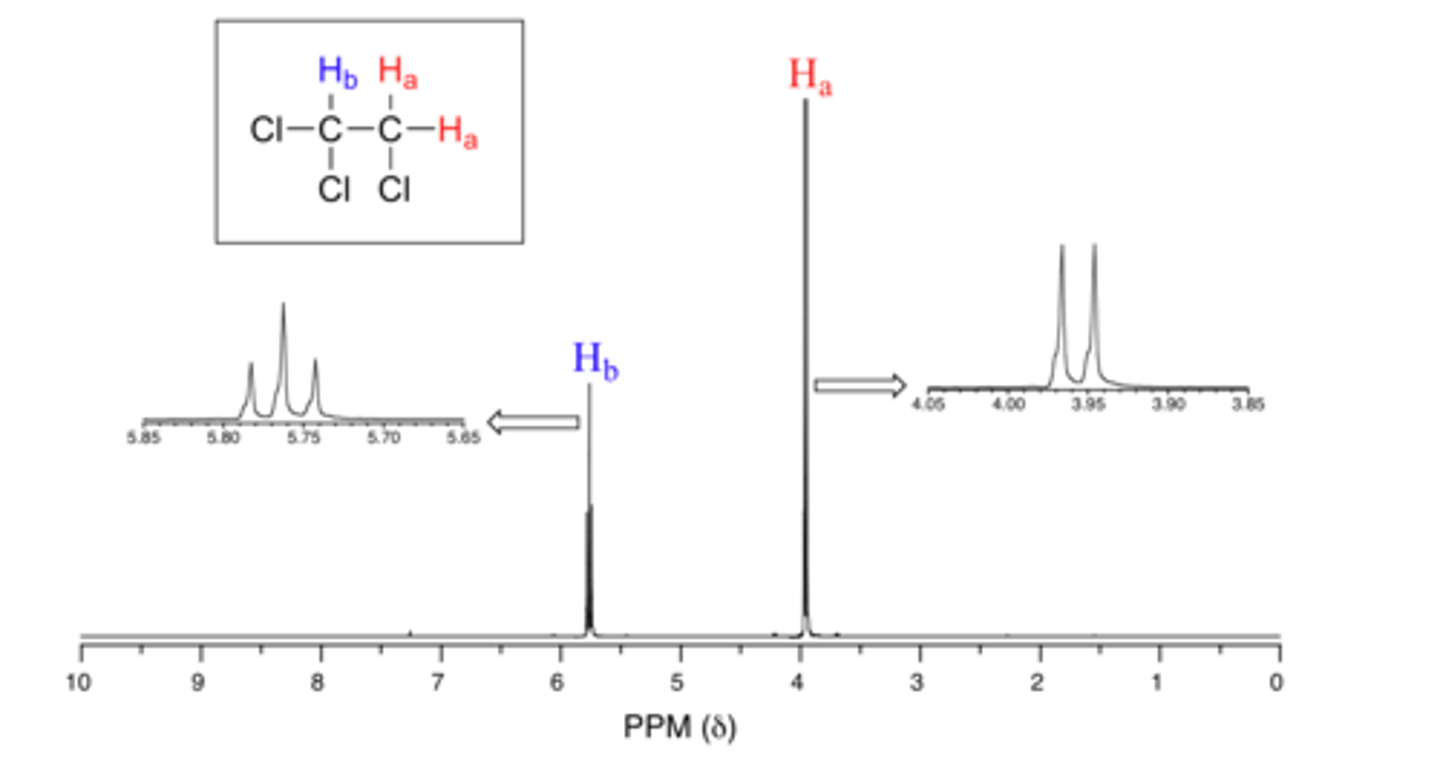
What rule/equation is used to determine the number of peaks when Splitting occurs?
Peaks = n+1
n = number of neighboring equivalent protons.
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
CRB Match the number of peaks with the number of Hydrogens associated with each peak pattern, assuming there is splitting occurring.
I. Triplet
II. Doublet
III. Quintet
(A) 1
(B) 2
(C) 3
(D) 4
I. Triplet (B) 2
II. Doublet (A) I
III. Quintet (D) 4
CRB True or false? If you see a singlet, there cannot be any splitting of that Hydrogen.
True. If you see a singlet, there cannot be any splitting of that Hydrogen.
CRB You are given a Proton NMR for a compound C2H4OCl2 and notice that there is a singlet far downfield from the other signals. Which of the following is the best explanation?
(A) There are two Hydrogens here that are not splitting each other.
(B) There are two Hydrgoens here that are splitting each other.
(C) There is a single Hydrogen that is surrounded by Electron Donating Groups.
(D) There is a single Hydrogen that is surrounded by Electron Withdrawing Groups.
(D) There is a single Hydrogen that is surrounded by Electron Withdrawing Groups.
The fact that this Hydrogen is downfield, along with the many electronegative atoms in this formula, suggest that this is a single Hydrogen that is surrounded by Electron Withdrawing Groups.
What is Geminal Coupling?
Geminal Coupling refers to the idea that two hydrogens on the same carbon can split one another if they are fixed in different chemical environments.
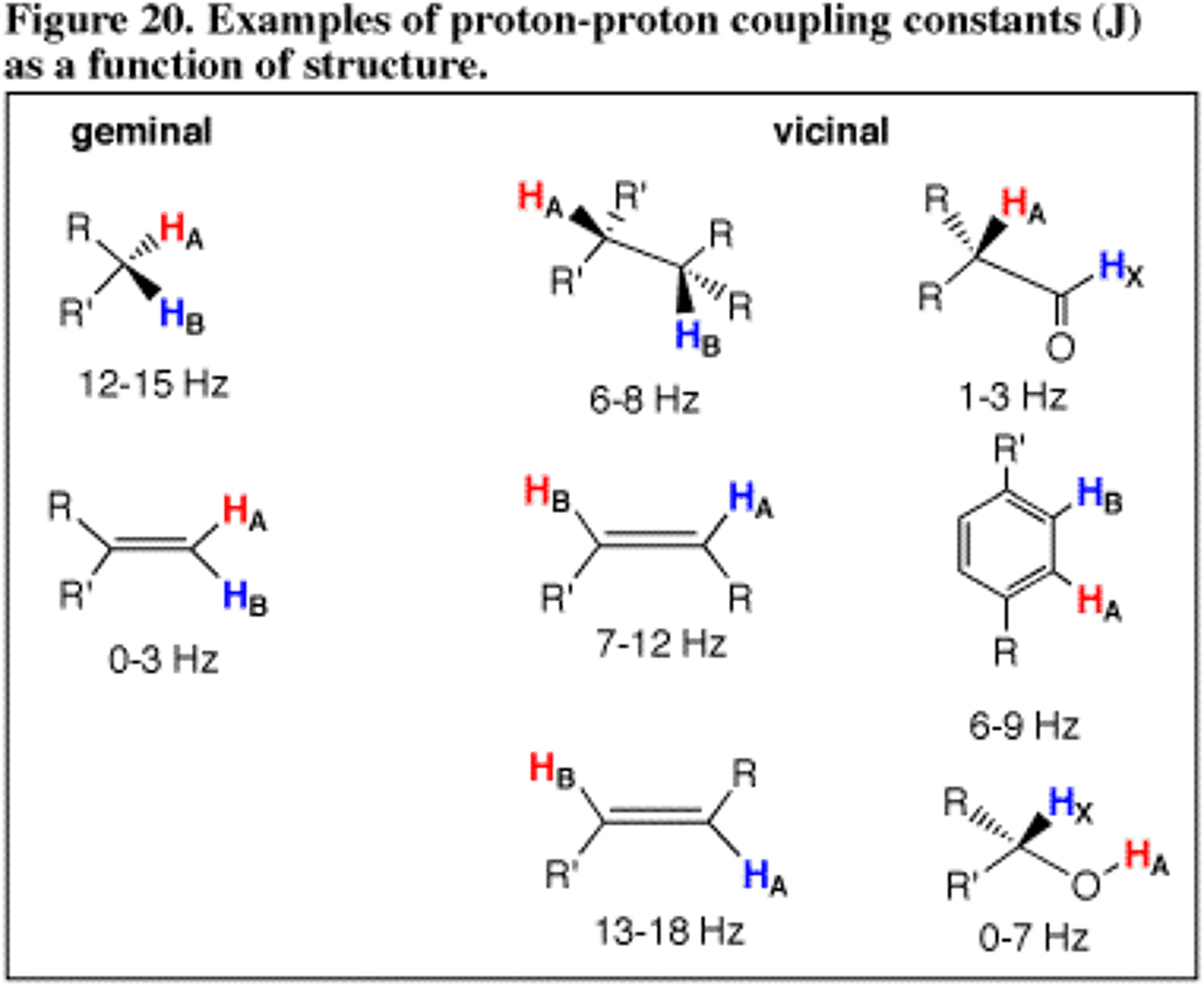
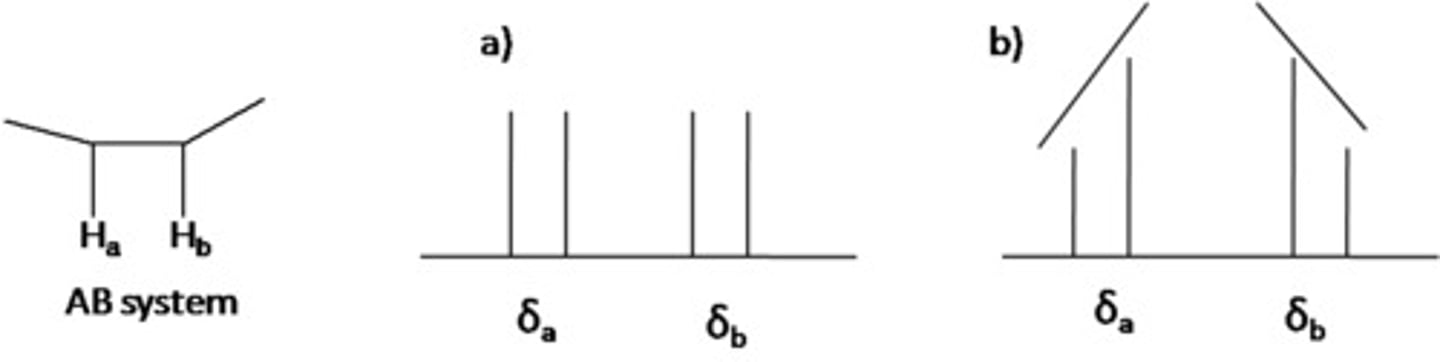
You observe a doublet and one peak within the doublet is higher than the other. What is the significance of this?
The proton that is causing the splitting of that doublet must be on the same side as the higher peak. The doublet is "leaning" toward the proton that caused the splitting. As in the image, you would expect to see (b) and not (a) in your NMR.
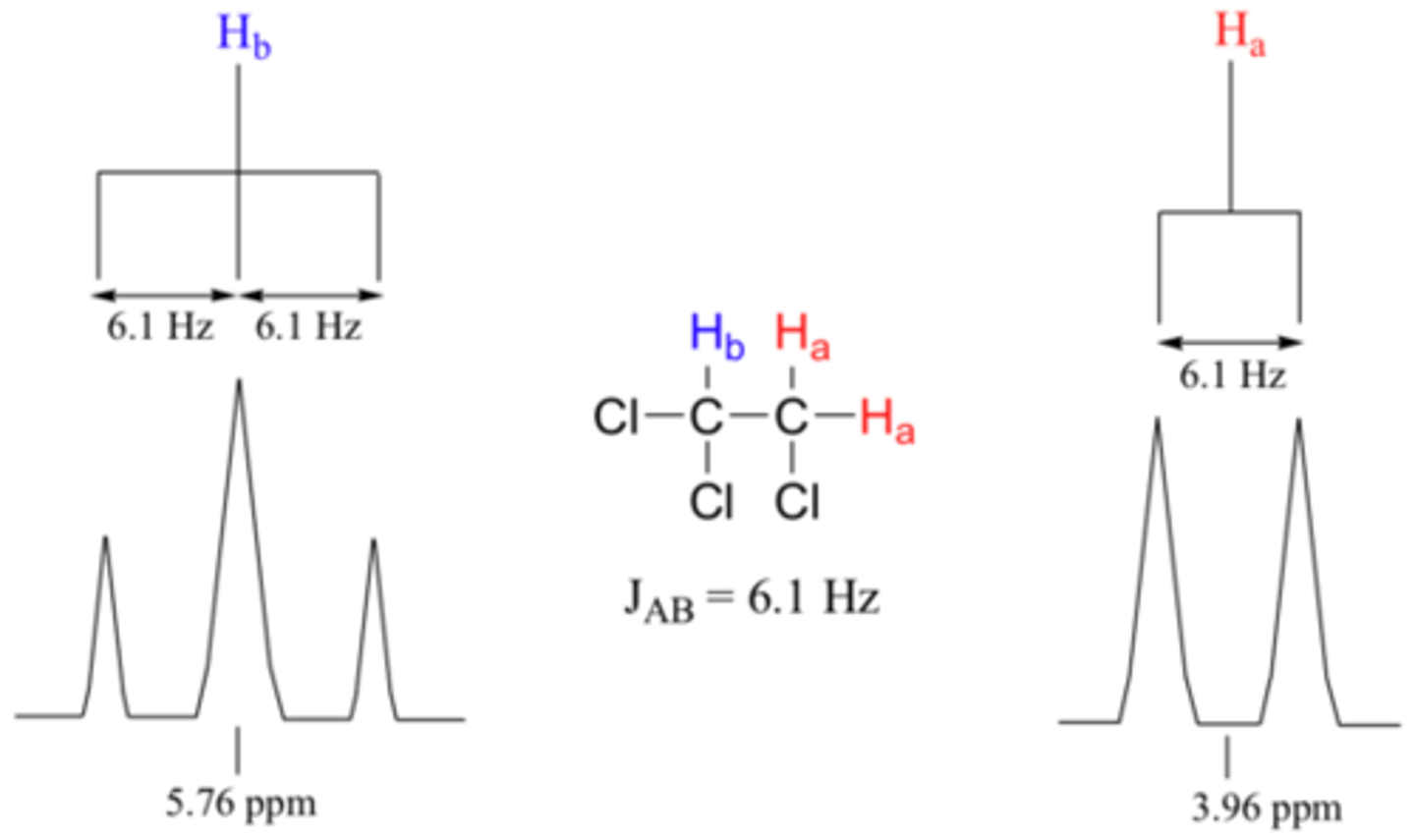
What information does a Coupling Constant tell you?
It tells you how far apart peaks should be when splitting occurs.
A proton is next to two nonequivalent protons with coupling constants of 3.4 Hz and 7.5 Hz. How many peaks would you expect to see?
(A) 2
(B) 3
(C) 4
(D) 5
(C) 4
The proton's peak will be split into two peaks that are 7.5 Hz apart and then each of those peaks will be split into two peaks that are 3.4 Hz apart. Because none of the peaks overlap, 4 peaks are observed for this one proton.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB Which of the following compounds could appear on an NMR?
I. C-14
II. O-15
III. S-32
(A) I only
(B) II only
(C) I and III only
(D) I, II and III
(B) II only
To appear on an NMR, an atom must have an odd mass number, an odd atomic number, or both. Only O-15 meets either of these criteria.
CRB Based on the previous description of what makes molecules sensitive to NMR, what is the Carbon Isotope used for Carbon NMR?
Carbon-13, since it has an odd mass number.
CRB C-13 NMR also uses ppm as its units. Rank the following groups from the largest to smallest shifts in ppm.
I. Alkynes
II. Alkenes
III. Aldehydes
IV. Carboxylic Acids
V. Aromatics
(A) I > II > III > IV > V
(B) II > I > III > V > IV
(C) III > IV > V > II > I
(D) IV > III > V > I > II
(C) III > IV > V > II > I
The order from largest to smallest shifts are:
1. Aldehydes (190-200 ppm)
2. Carboxylic Acids (175-185 ppm)
3. Aromatics (110-170 ppm)
4. Alkenes (100-150 ppm)
5. Alkynes (65-85 ppm)
Values are pulled from this table: https://www2.chemistry.msu.edu/courses/cem251/SS13_HOVIG/Spectroscopy%20tables.pdf
What equation can be used to calculate a Hydrogen Deficiency Index?
HDI = ((2n+2) - x) / 2
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
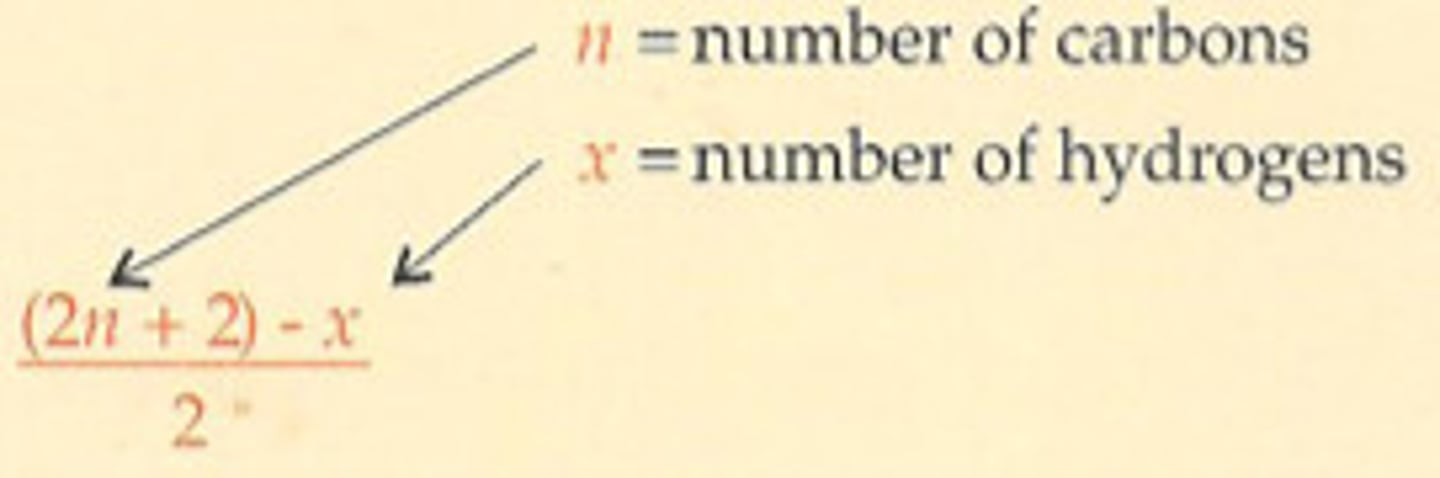
CRB Which of the following is a common synonym for the Hydrogen Deficiency Index that also gives insight to how it is applied?
(A) Order of Magnitude
(B) Absolute Value of Integration
(C) Proton Splitting Index
(D) Degree of Unsaturation
(D) Degree of Unsaturation
Knowing the degrees of Unsaturation of a molecule will tell you how many pi bonds or ring structures that you have in the compound.
What is the Hydrogen Deficiency Index for Benzene? What does this tell you?
HDI = ((2n+2) - x) / 2
HDI = (((2⋅6)+2) - 6) / 2
HDI = (14 - 6) / 2
HDI = 4
The Hydrogen Deficiency Index for Benzene is equal to 4. This means that Benzene contains 4 pi bonds and/or rings.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
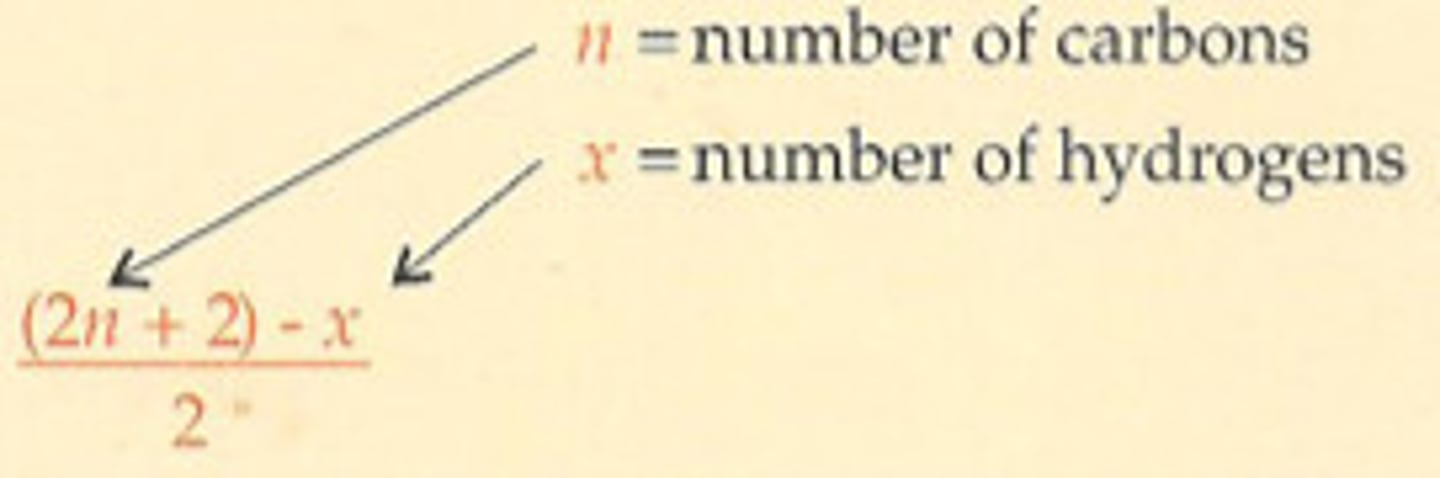
How are Nitrogens taken into account for our Hydrogen Deficiency Equation? Halogens?
Nitrogens (N) are added to the numerator, and Halogens (X) are subtracted from the numerator:
HDI = ((2n+2) - x + N - X) / 2
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
Practice your newfound NMR skills using the H-NMR practice problems found here (the ones with the ** are the best since they offer a step-by-step solution): http://www-usr.rider.edu/~grushow/nmr/NMR_tutor/selftests/problems_fs_start.html
The answers are found in the same place.