Physics II Lesson 5: Spectroscopy Part II
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Which will have a higher wavenumber? C-O double bond without resonance or a C-O double bond with resonance? Why?
C-O double bond without resonance will have a higher wavenumber than a C-O double bond with resonance because resonance will cause the bond to have partial single bond character.
CRB Fill in the blanks: Because a double bond is ______________ than a single bond, it requires (a) ______________ to vibrate the bond, which means there is ___________ energy needed to cause that vibration.
(A) Longer, more force, more
(B) Stiffer, less force, less
(C) Stiffer, higher wavenumber, more
(D) More elastic, lower wavenumber, less
(C) Stiffer, higher wavenumber, more
Because a double bond is Stiffer than a single bond, it requires a higher wavenumber to vibrate the bond, which means there is More energy needed to cause that vibration.
CRB Which of the following would be appropriate examples to highlight the point that stronger, stiffer bonds need a higher wavenumber to vibrate the bond?
I. An Ether requires a higher wavenumber than an Alcohol
II. A Ketone's Carbon-Oxygen bond requires a higher wavenumber than an Ether
III. sp-C-H bonds require a higher wavenumber than an sp3-C-H bond.
(A) I only
(B) I and II only
(C) II and III only
(D) I, II and III
(C) II and III only
Each of the following statements are true:
II. A Ketone's Carbon-Oxygen bond requires a higher wavenumber than an Ether
III. sp-C-H bonds require a higher wavenumber than an sp3-C-H bond.
An Ether is a C-O bond that absorbs in the fingerprint region, whereas alcohols absorb broadly around 3100 cm^-1, a much higher wavenumber.
Which will have the higher wavenumber for the C-O Double Bond? An Acyl Chloride or a Carboxylic Acid? Why?
An Acyl Chloride will have a higher wavenumber than a Carboxylic Acid because the Chlorine Atom will draw more electron density towards it due to its higher electronegativity, resulting in greater double bond character at the C-O double bond.
CRB True or false? If the Hydrogen in the Carboxylic Acid were replaced by Tritium (Hydrogen with an atomic mass of 3), then the O-T bond would have a higher wavenumber than the original O-H bond.
False. If the Hydrogen in the Carboxylic Acid were replaced by Tritium (Hydrogen with an atomic mass of 3), then the O-T bond would have a lower wavenumber than the original O-H bond.
This is because a more massive atom will vibrate more slowly and at lower frequencies.
CRB Kyrie is having a tough time remembering that a heavier atom will have a lower stretching frequency (wavenumber). Can you think of an easier to remember example?
The easiest way to remember this is by comparing C-H and C-C bonds. C-H bonds have a wavenumber near 3000 cm^-1, whereas C-C bonds are in the fingerprint region below1500 cm^-1.
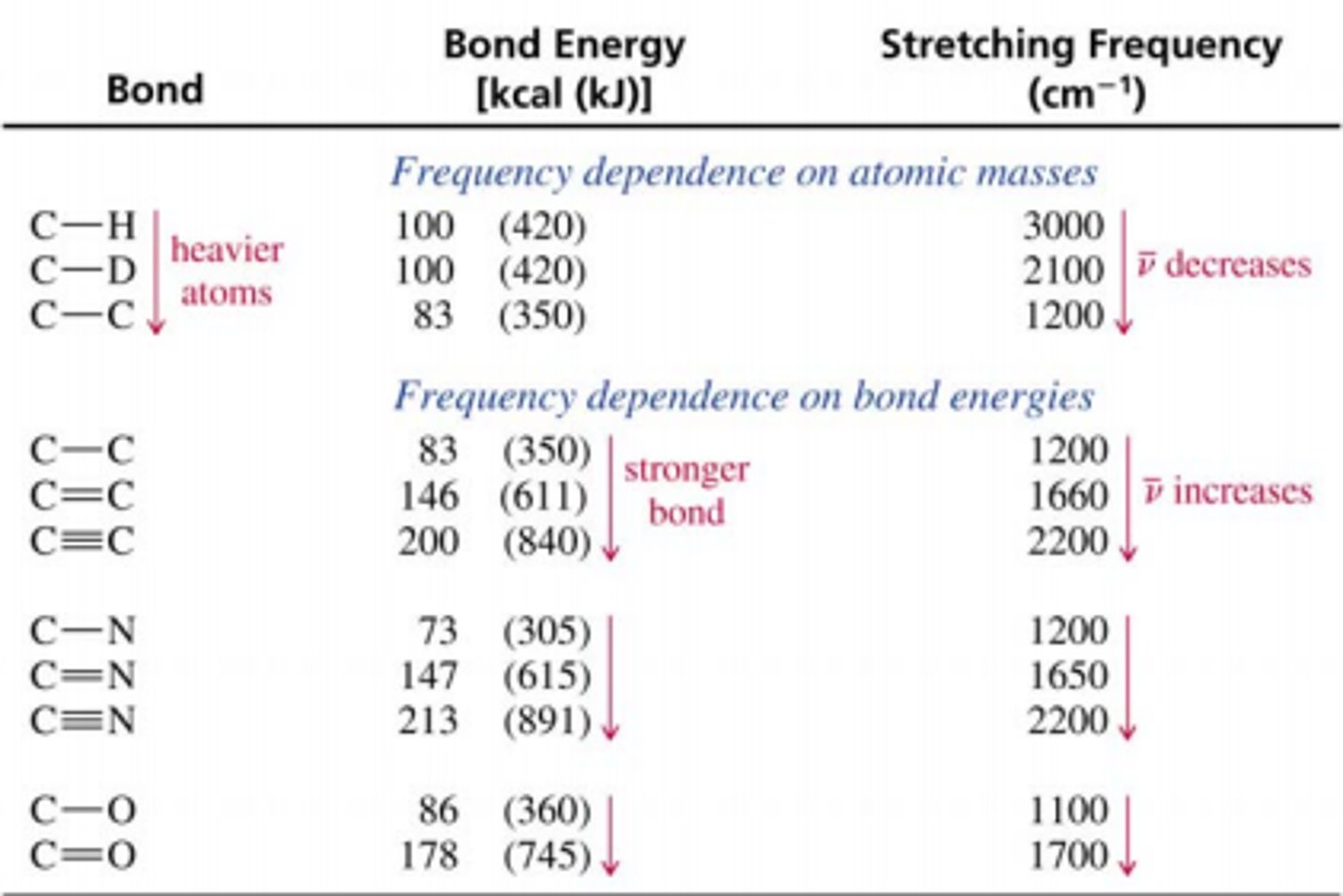
CRB Since having a heavier atom leads to a lower wavenumber, what type of relationship do atomic weight and wavenumbers have?
(A) Linear
(B) Positive Correlation
(C) Exponential
(D) Negative Correlation
(D) Negative Correlation
Since an increase in one factor leads to a decrease in the other, this is a negative correlation.
CRB Since a stiffer bond leads to a higher wavenumber, what type of relationship do wavenumbers and bond stiffness have?
(A) Linear
(B) Positive Correlation
(C) Exponential
(D) Negative Correlation
(B) Positive Correlation
Since an increase in one will lead to an increase in the other, this is a positive correlation.
Practice identifying compounds based on their IR Spectra with the four practice problems found here: https://www.masterorganicchemistry.com/2016/11/29/ir-spectroscopy-some-simple-practice-problems/
Answers found at the bottom of the page here: https://www.masterorganicchemistry.com/2016/11/29/ir-spectroscopy-some-simple-practice-problems/
What is on the x-axis for a UV/Vis Spectroscopy graph? What about the y-axis?
On the x-axis, you will find the wavelength. On the y-axis, you will find the absorbance.
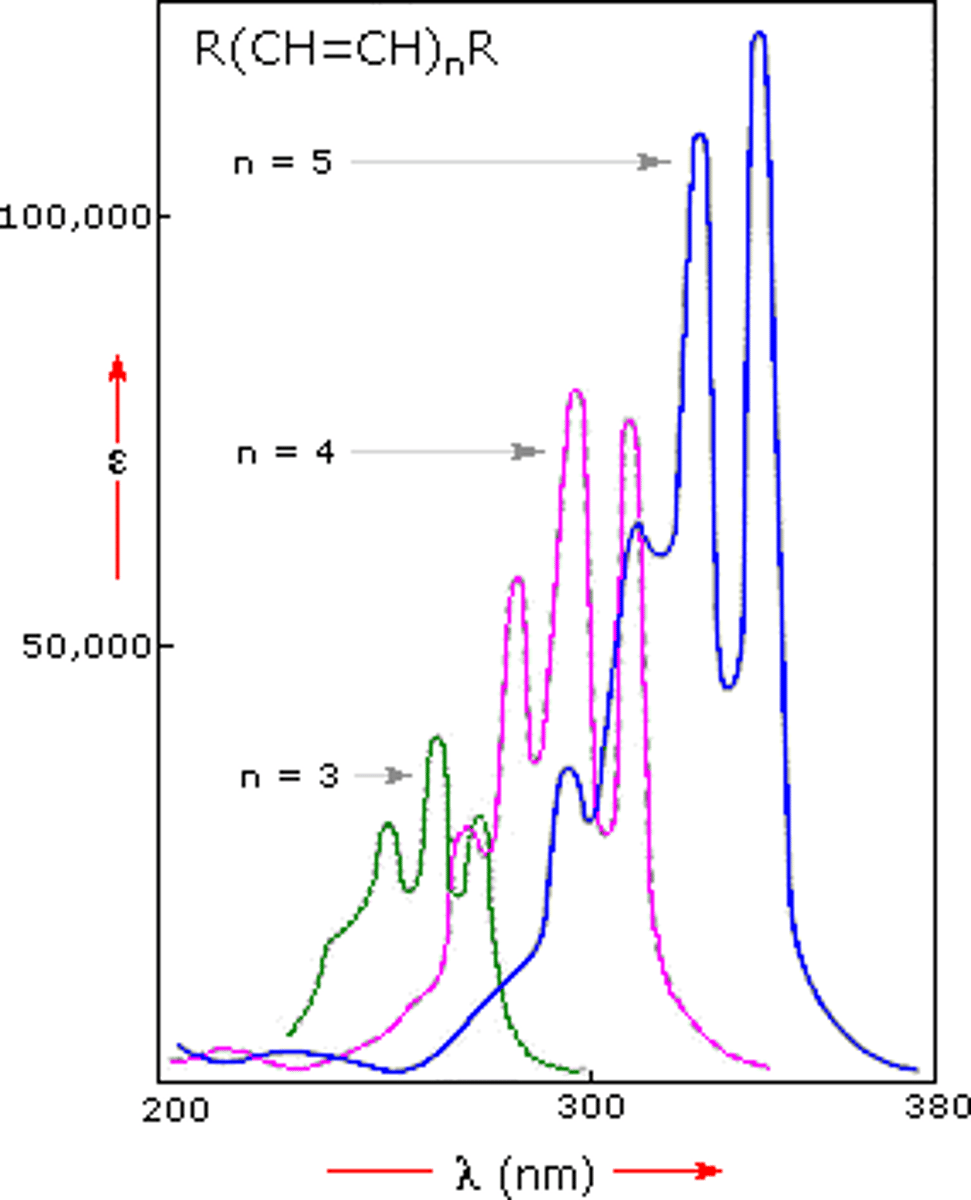
CRB Fill in the blanks: UV/Vis Spectroscopy uses ____________ wavelengths than IR to excite the bonds, meaning that those bonds must be ___________.
(A) Shorter, Weaker
(B) Longer, Weaker
(C) Shorter, Stronger
(D) Longer, Stronger
(C) Shorter, Stronger
UV/Vis Spectroscopy uses Shorter wavelengths than IR to excite the bonds, meaning that those bonds must be Stronger.
CRB Which of the following types of molecules would you expect to measure with UV/Vis Spectroscopy?
I. Transition Metal Complexes
II. Aromatic Molecules
III. Other Highly Conjugated Molecules
(A) II only
(B) I and II only
(C) II and III only
(D) I, II and III
(D) I, II and III
Each of the following would need UV/Vis Spectroscopy to be measured:
I. Transition Metal Complexes
II. Aromatic Molecules
III. Other Highly Conjugated Molecules
CRB True or false? A more conjugated compound will be even more stable, so it will need more energy (shorter wavelengths) to be excited than less conjugated compounds.
False. A more conjugated compound will be more stable than less conjugated systems if one electron pair is excited above ground state, so it can use less energy (longer wavelengths) to be excited.
Draw the ground-state electron configuration for Butadiene. Then draw the resulting configuration upon absorption of UV light.
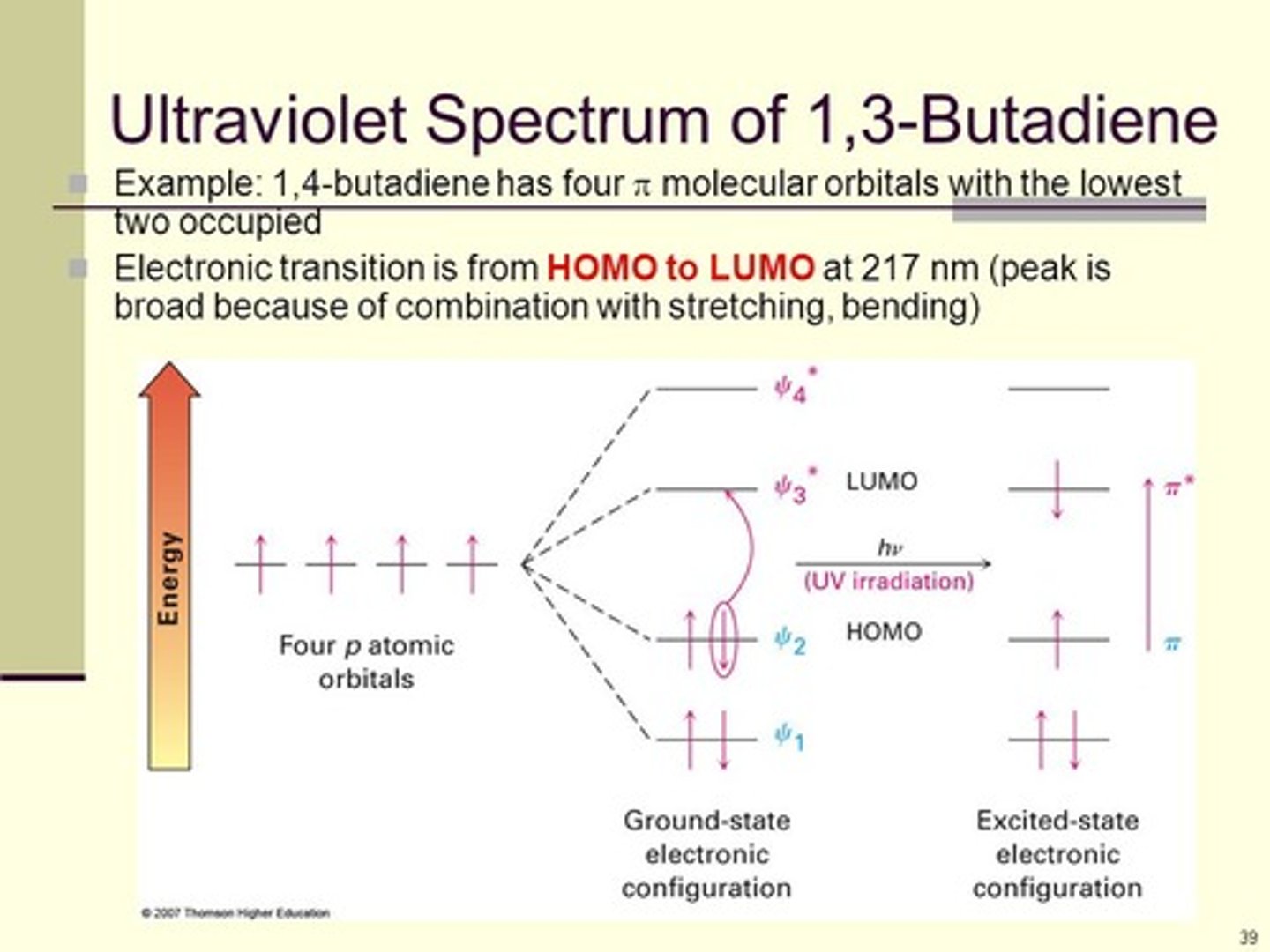
CRB Fill in the blanks: In UV/Vis Spectroscopy, electrons are excited from the _____________ to the _______________.
(A) HOMO, LOMO
(B) HOMO, LUMO
(C) HUMO, LUMO
(D) HUMO, LOMO
(B) HOMO, LUMO
Highest Occupied Molecular Orbital, Lowest Unoccupied Molecular Orbital
In UV/Vis Spectroscopy, electrons are excited from the HOMO to the LUMO.
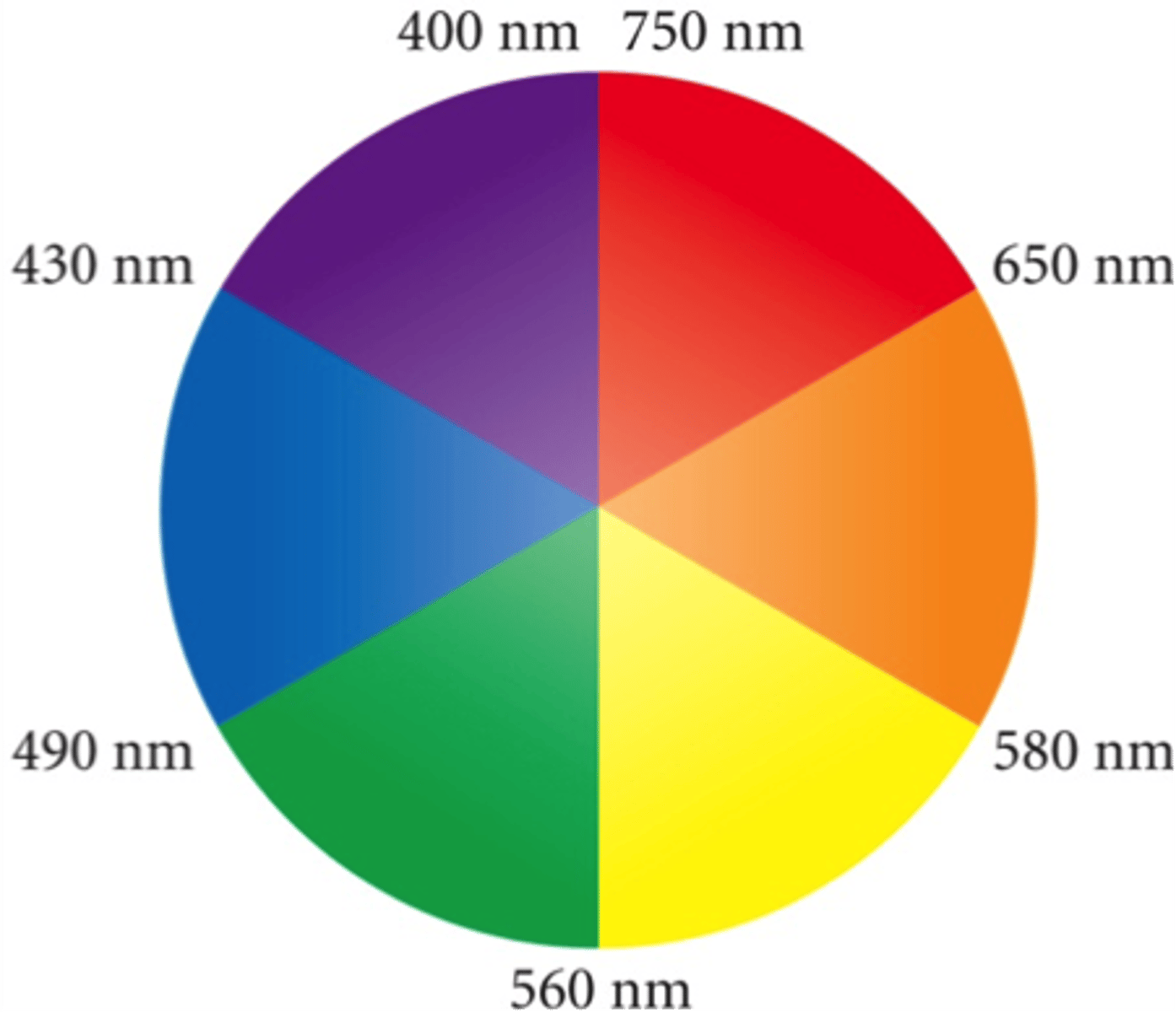
What wavelengths correspond to visible light?
From highest to lowest: ROYGBV.
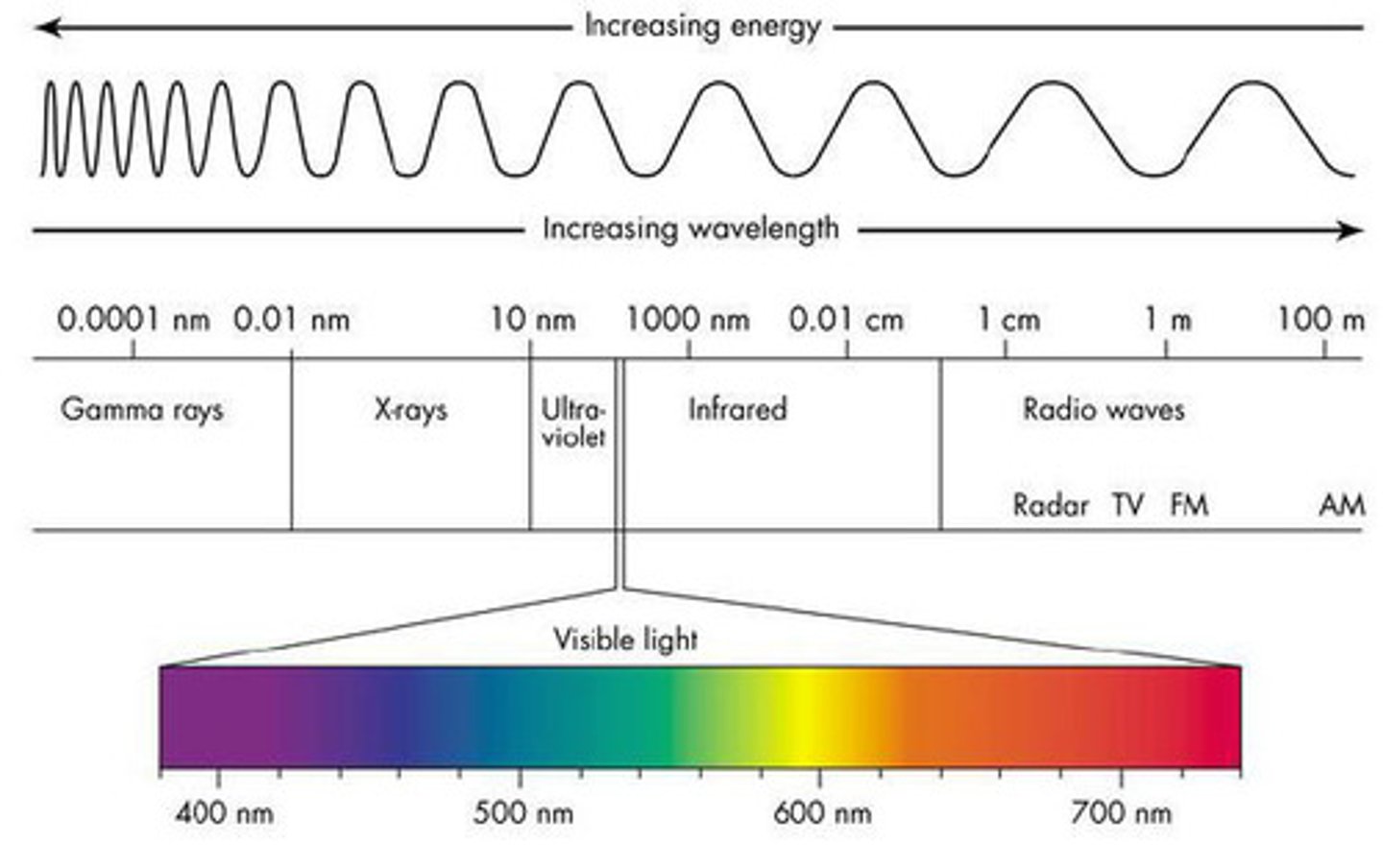
You look outside and are in awe at the fall colors on all the tree. You especially love a tree with bright red leaves. Which of the following is true about the leaves on that tree?
(A) They absorb only red light.
(B) They absorb every color except red light.
(C) They absorb every color, including red light.
(D) They absorb multiple colors that when mixed result in the complimentary color of red light.
(B) They absorb every color except red light.
Instead of absorbing red light, the leaves are reflecting the red light, which will be registered by your eyes.

A certain compound has a peak on an IR-Spectrum around 530 nm (green). What color do you expect this compound to be?
You'd expect the compound to be red since it's complimentary color, green, is what is being absorbed.
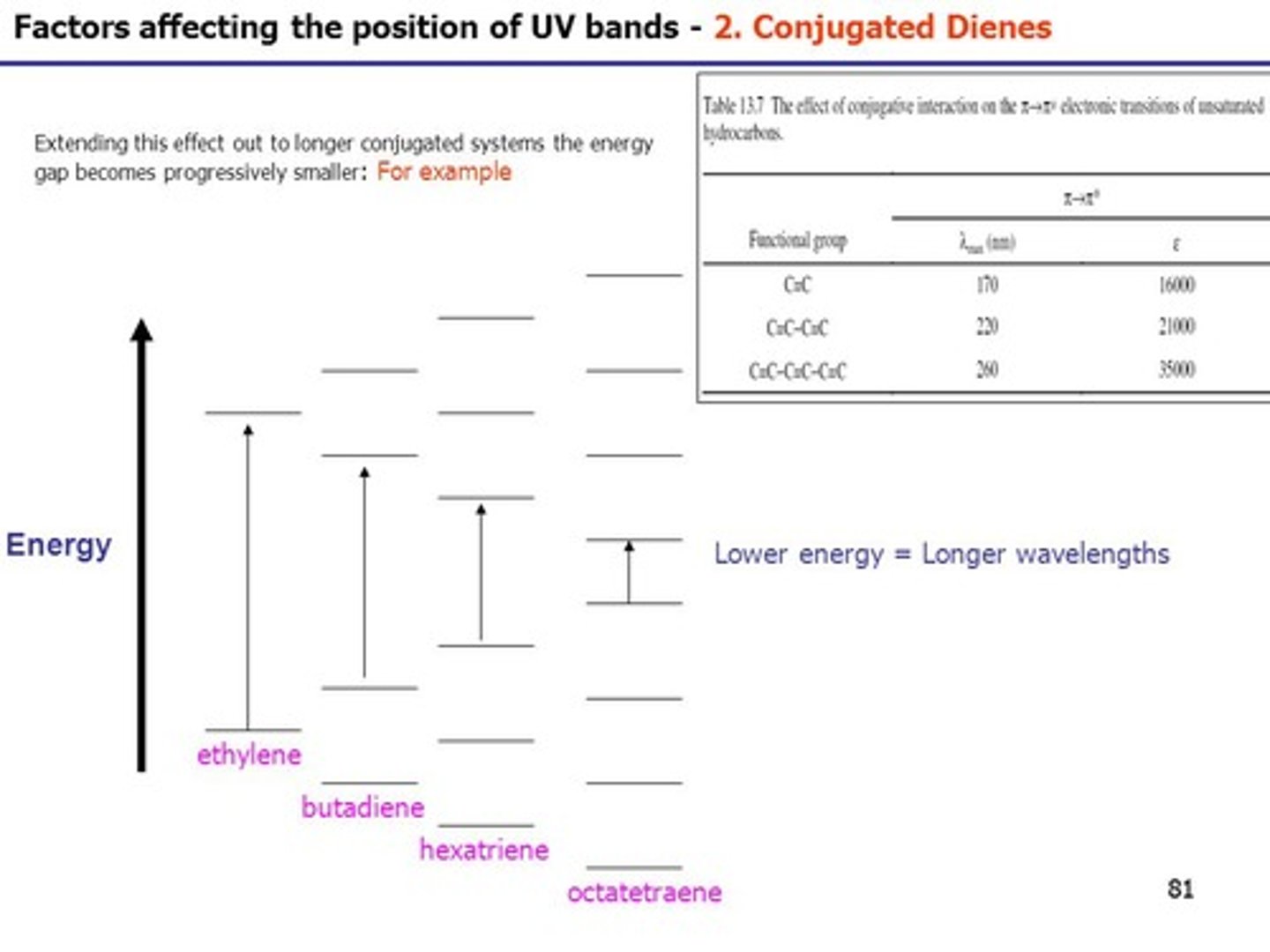
Put the following in order of lowest to highest wavelength for absorbance:
I. Ethene
II. Butadiene
III. Hexatriene
(A) I < II < III
(B) I < III < II
(C) III < II < I
(D) III < I < II
(A) I < II < III
In order of lowest to highest wavelength for absorbance: Ethene < Butadiene < Hexatriene. This is due to the fact that Ethene's MO's are farthest apart and thus absorb a higher energy of light, corresponding to a lower wavelength.
Thus, what is the relationship between wavelength and conjugation? How does this relate to color?
The greater the conjugation, the higher the wavelength. The higher the wavelength, the less likely for the wavelength to be UV light and more likely to be in the visible light spectrum.
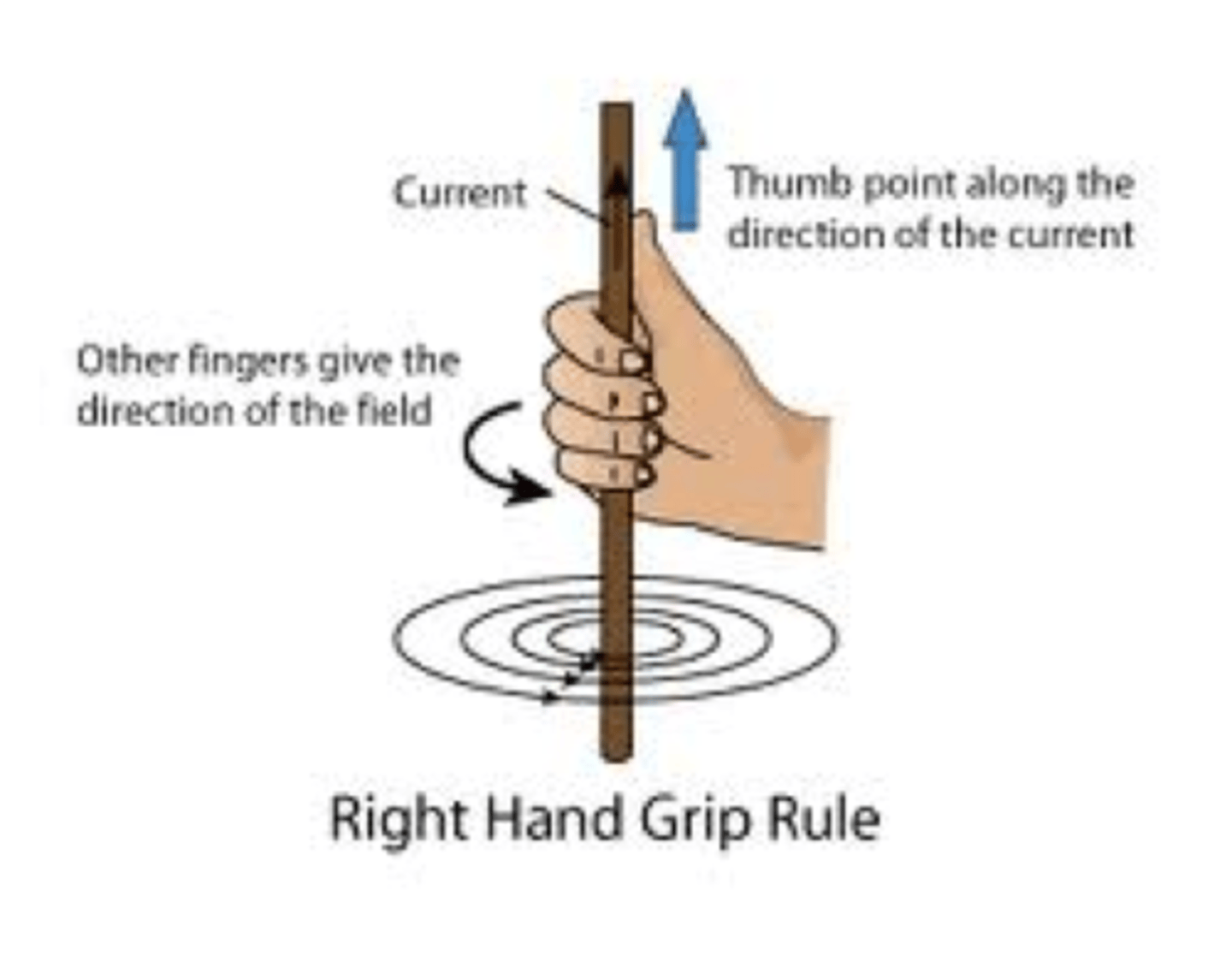
A Proton can be viewed as a tiny magnet, which means it has a magnetic field. You are looking down on a proton and notice that it is spinning in a counter-clockwise direction. In which direction does the magnetic field point?
(A) Counter-clockwise
(B) Clockwise
(C) Away from you
(D) Towards you
(D) Towards you
This can be determined using the right-hand rule.
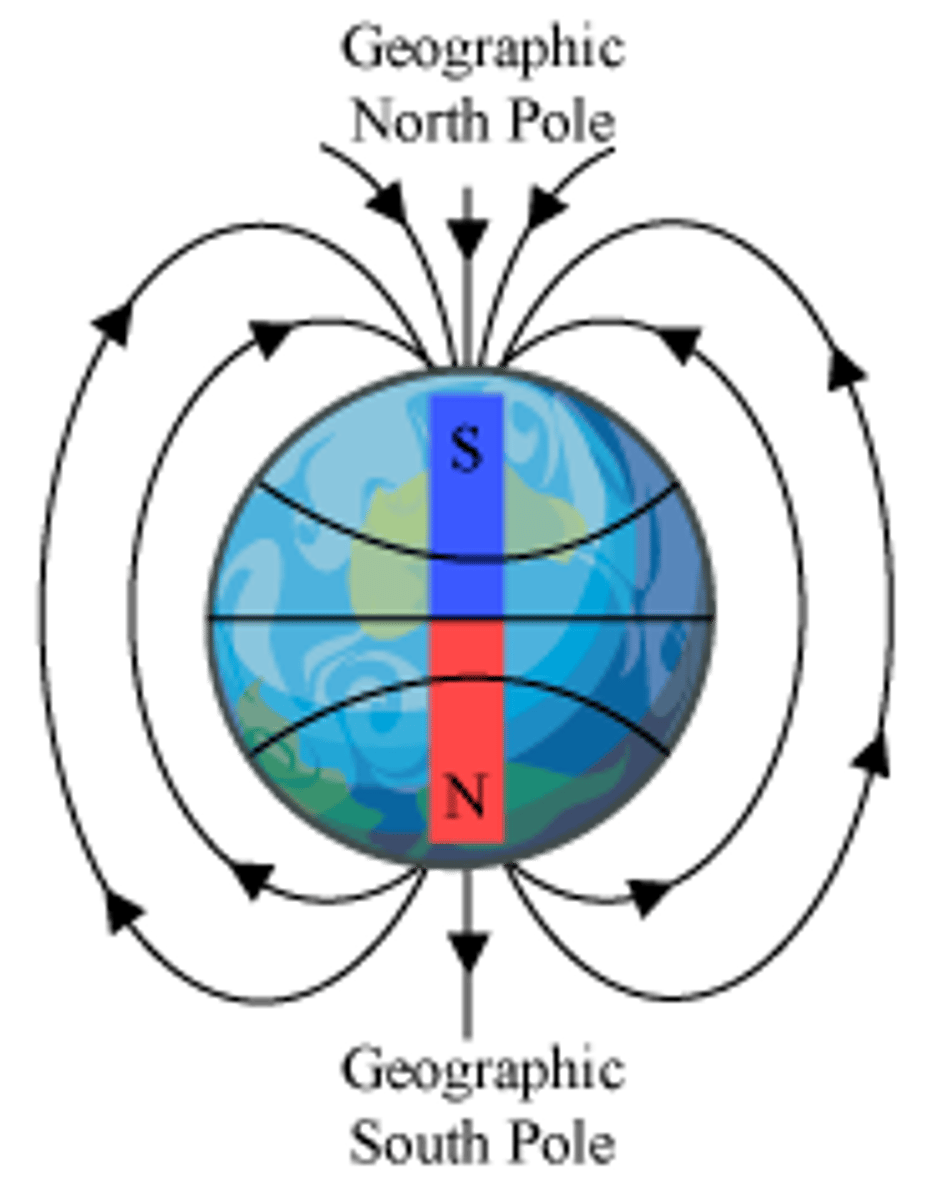
The north pole of a magnet points toward the magnetic north or south pole of the earth?
The north pole of a magnet points toward the magnetic south pole of the earth and the geographic north pole.
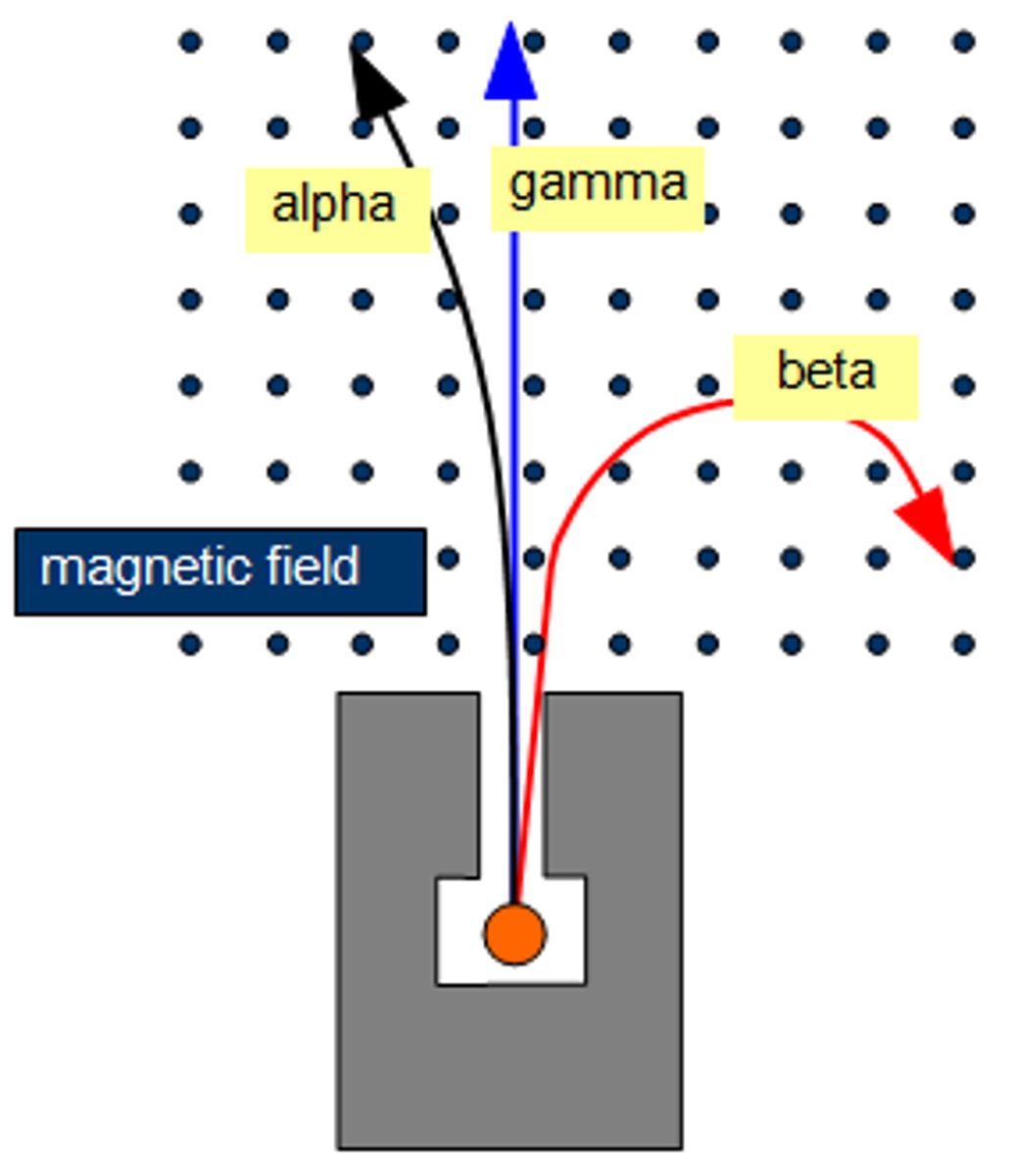
The external magnetic field (B₀) is pointing in an upward direction. In which direction does the magnetic field of a proton point in the α state? What about the β state? Which is the higher energy state?
α state - The magnetic field of the proton will point upwards, in line with B₀.
β state - The magnetic field of the proton will point downwards, out of line with B₀. This is the higher energy state.
You increase the strength of the external magnetic field (B₀). What happens to the energy difference between the α and the β state?
The energy difference between the two states will increase.
What is seen on the x-axis of an NMR Spectra? What about the y-axis? What does each give you information about.
On the x-axis is the chemical shift (ppm), which corresponds to frequency, which corresponds to the strength of the magnetic field where that H or C atom is. This will let you know what functional groups are nearby.
On the y-axis is the intensity of the signal. This will tell you about how many H's or C's are in that same magnetic environment.
Electrons floating around a Hydrogen atom will create an induced magnetic field (βin). How does this relate to the external magnetic field (B₀) and the overall, effective magnetic field (βeff)?
βin will combine with B₀ and result in the overall βeff. If βin opposes B₀, then the overall relationship might be summarized as follows:
βeff = B₀ - βin
Electrons are said to "shield" the proton. What does this mean?
To say that electrons "shield" the proton is to say that the electrons create a βin that opposes the B₀, which will decrease βeff. The overall magnetic field experienced by the proton is lowered because they were shielded by the electrons.
CRB Being next to which of the following types of groups would most shield a proton?
(A) Hydrogen
(B) EDG
(C) EWG
(D) Not enough information
(B) EDG
Since having electrons around decreases the overall magnetic field experienced, an Electron Donating Group will be most effective in shielding!
Proton A is more deshielded than Proton B. Which proton will appear farther downfield in an NMR Spectra?
(A) Proton A because going from the α to the β state will take more energy than with Proton B.
(B) Proton A because going from the α to the β state will take less energy than with Proton B.
(C) Proton B because going from the α to the β state will take more energy than with Proton A.
(D) Proton B because going from the α to the β state will take less energy than with Proton A.
(A) Proton A because going from the α to the β state will take more energy than with Proton B.
Proton A is more deshielded, resulting in a greater βeff, which results in a greater energy difference between the α and the β state, which results in a greater energy required. Greater energy corresponds to a higher frequency, which is farther downfield in an NMR Spectra.
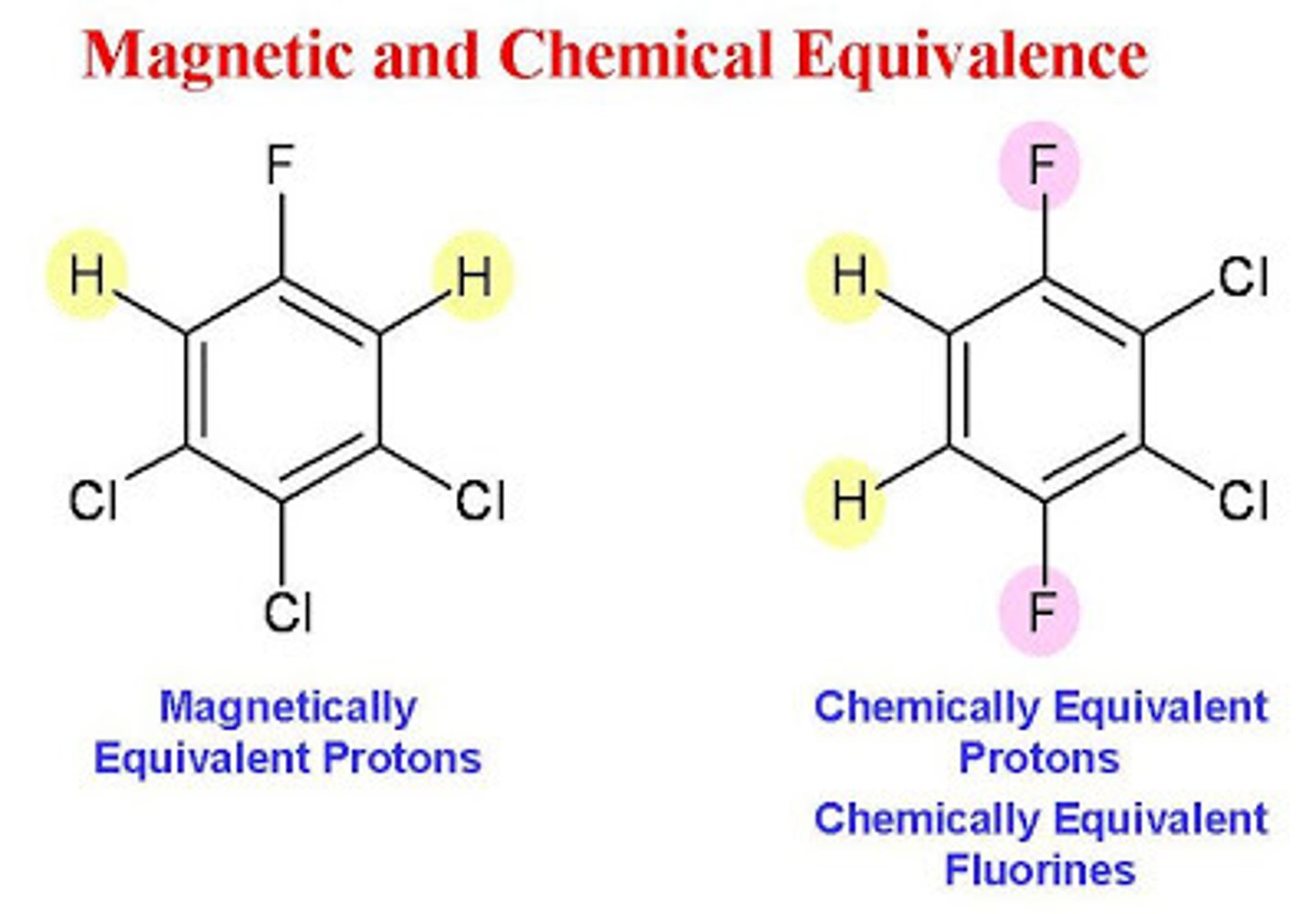
When speaking about NMR, what does it mean to say that protons are "chemically equivalent"? How does this relate to how they will appear in an NMR Spectra?
To say that protons are "chemically equivalent" is to say that the protons are in the same magnetic environment. These protons will therefore correspond to the same, single peak in an IR Spectra.
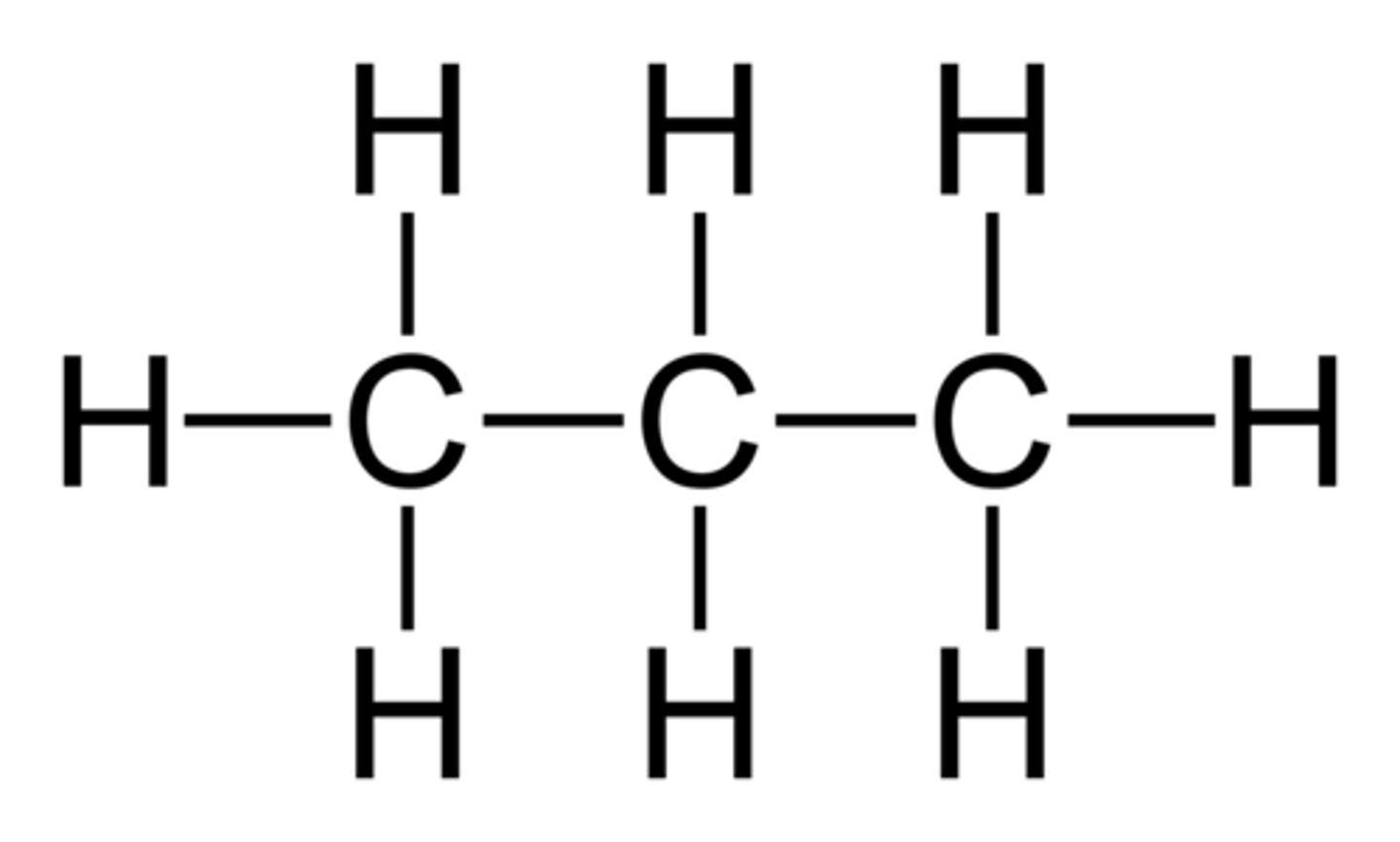
How many signals would you expect to result from propane on an NMR Spectra?
(A) 1
(B) 2
(C) 3
(D) 4
(B) 2
Propane has 8 protons total, with 2 being in one magnetic environment and 6 being in another. Because there are two magnetic environments, propane will display two peaks in an NMR Spectra.
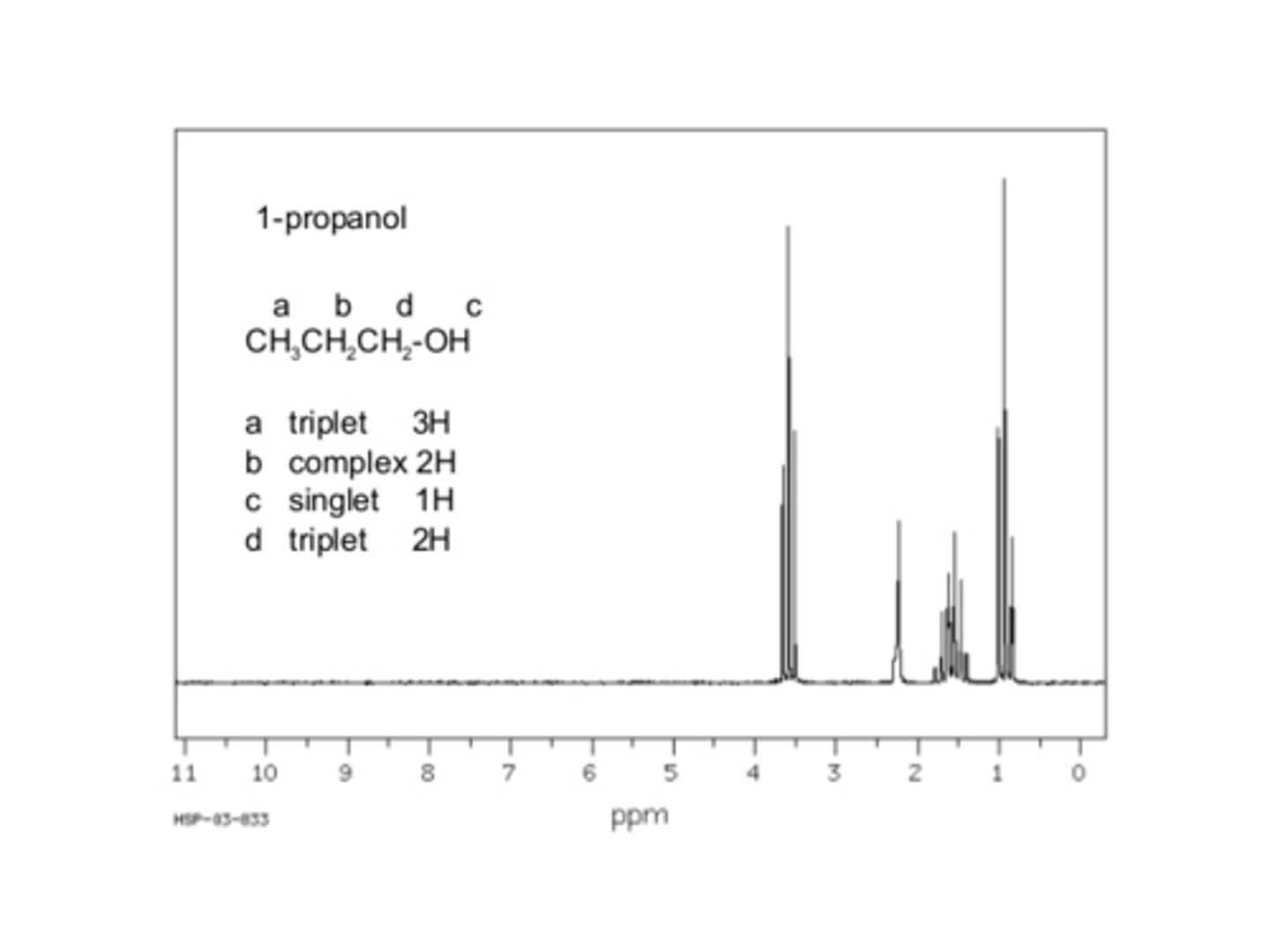
How many signals would you expect to result from 1-propanol on an NMR Spectra?
(A) 1
(B) 2
(C) 3
(D) 4
(D) 4
1-Propanol has 8 total protons in four different environments: 2 on carbon 1, 2 on carbon 2, 3 on carbon 3, and the final one bound to the oxygen. Because there are 4 magnetic environments, propanol will display 4 peaks on an NMR spectra.
What is the structure of Tetramethylsilane (TMS)? Where will you see its signal in an NMR Spectra? Why is this significant?
TMS has a signal at 0 Hz. It is useful to allow us to know where 0 is on the NMR Spectra. Think of it like a standard.
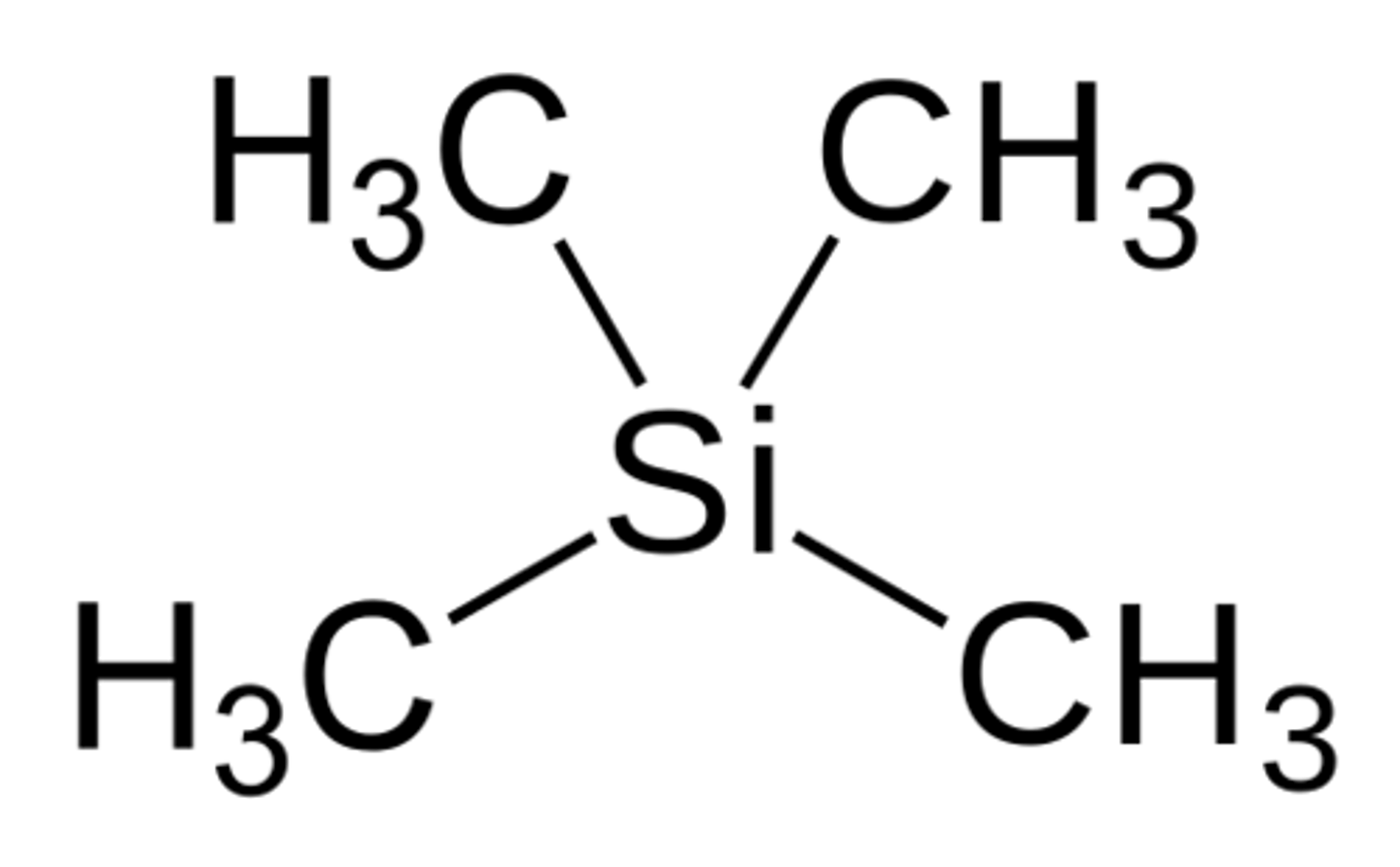
CRB Which of the following would best categorize the methyl groups of TMS?
I. Electron Donating Group
II. Electron Withdrawing Group
III. Having Chemically equivalent Hydrogens
(A) I only
(B) II only
(C) I and III only
(D) II and III only
(C) I and III only
Each of the hydrogens in TMS are chemically equivalent, and the Methyl Groups are known as EDGs.
What equation will allow you to calculate the chemical shift (δ) for a proton when given its frequency?
δ = (f(proton) - f(protons in TMS))⋅10^6 / f(spectrometer)
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
You are doing an NMR. The frequency of the spectrometer is 300 MHz. The frequency of the hydrogen of interest is 643 Hz higher than the frequency of the hydrogens in TMS. What is the chemical shift for the hydrogen of interest?
(A) 4.69
(B) 3.16
(C) 2.14
(D) 1.77
(C) 2.14
δ = (f(proton) - f(protons in TMS))⋅10^6 / f(spectrometer)
δ = 643⋅10^6 / 300⋅10^6
δ = approx. 2 (actual: 2.14)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
Protons that are next to an electronegative atom will be more or less downfield than protons that are not? Why?
(A) More. This is because the protons will be more deshielded by the electronegative atom.
(B) More. This is because the protons will be less deshielded by the electronegative atom.
(C) Less. This is because the protons will be more deshielded by the electronegative atom.
(D) Less. This is because the protons will be less deshielded by the electronegative atom.
(A) More. This is because the protons will be more deshielded by the electronegative atom.
Protons that are next to an electronegative atom will be more downfield than protons that are not. This is because an electronegative atom will draw electron density away from the protons, making them deshielded.
What functional group would you expect to find between .5 and 2 ppm?
An alkane.
What functional group would you expect to find between 2 and 2.5 ppm?
A proton that is α to a carbonyl group.
What functional group would you expect to find between 2 and 5 ppm?
An Alcohol.
What functional group would you expect to find between 2.5 and 4.5 ppm?
A proton that is α to an electronegative atom.
What functional group would you expect to find between 4.5 and 6.5 ppm?
An Alkene.
What functional group would you expect to find between 6.5 and 8 ppm?
A proton that is part of an aromatic ring.
What functional group would you expect to find between 9 and 10 ppm?
An Aldehyde.
What functional group would you expect to find between 10 and 12 ppm?
A Carboxylic Acid.
Why do Alcohols have such a variable chemical shift (2 to 5 ppm)?
Alcohols exhibit hydrogen bonding, which will vary greatly depending on the compound.
Use hybridization to explain why alkenes are more deshielded than alkanes.
Alkenes are sp2-hybridized, giving them more s character than the sp3-hybridized orbitals of alkanes. sp2 orbitals have greater s-character and correspond with being closer to the nucleus and having greater electron density, which would result in greater deshielding of a nearby proton.