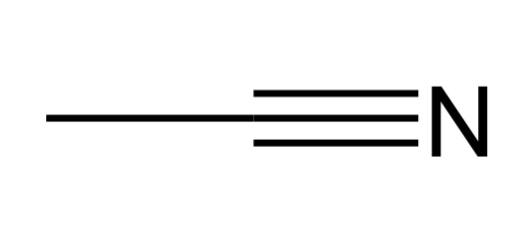Functional groups
1/21
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
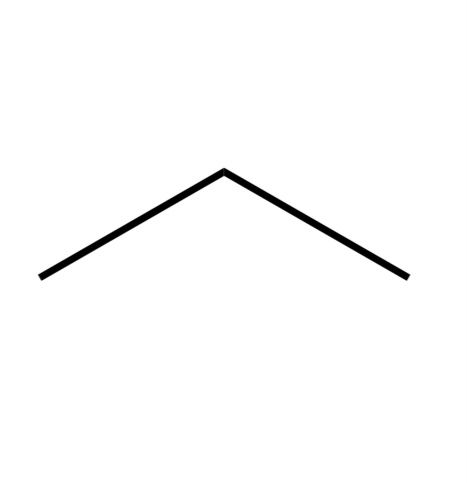
Alkane
A hydrocarbon consisting of only single bonds
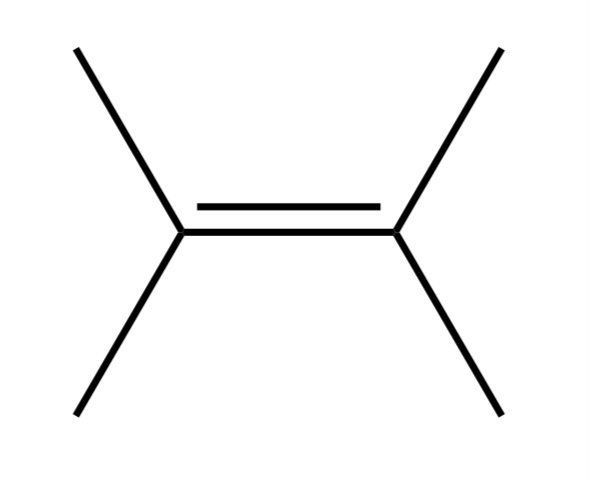
Alkene
A hydrocarbon that has a C=C bond
Alkyne
A hydrocarbon with a triple CC bond
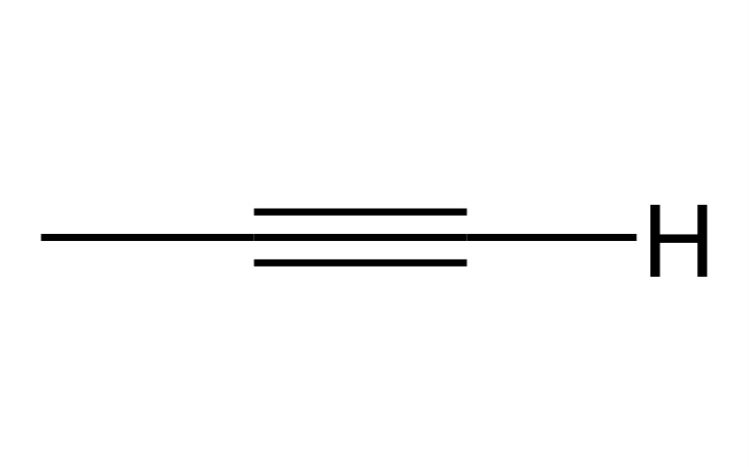
Benzene ring (phenyl)
A hydrocarbon ring with 6 carbons and 3 C=C bonds alternating
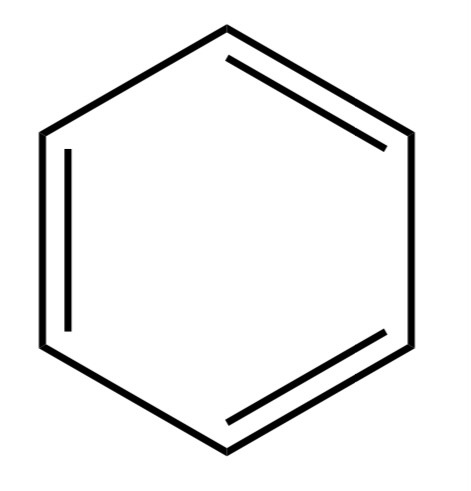
Isopropyl
An alkane with 3 carbons attached to a non hydrogen R griup
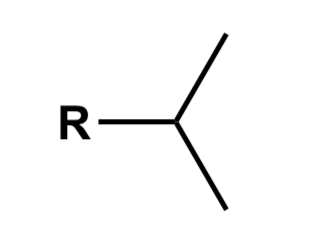
Tert-butyl
4 carbons attached a non hydrogen R group
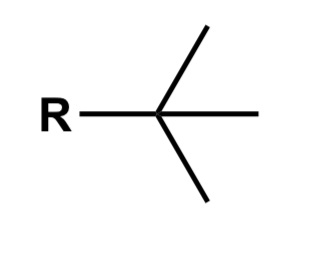
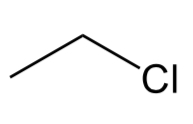
Alkyl halide
Alkane where one hydrogen is replaced w a halogen such as Cl
Amine
Nitrogen with 3 bonds and a lone pair
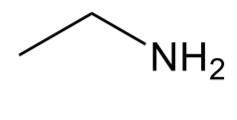
Alcohol
C-O-H group
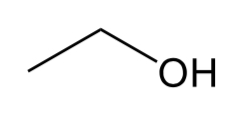
Ether
C-O-C group
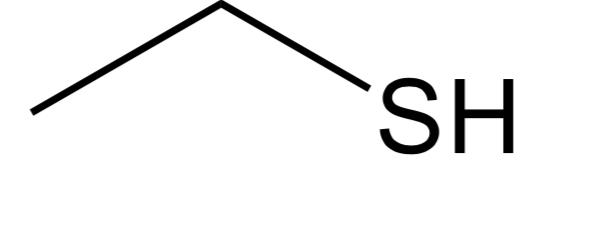
Thiol
C-S-H group
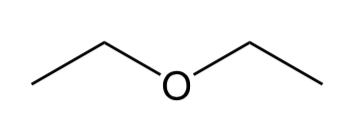
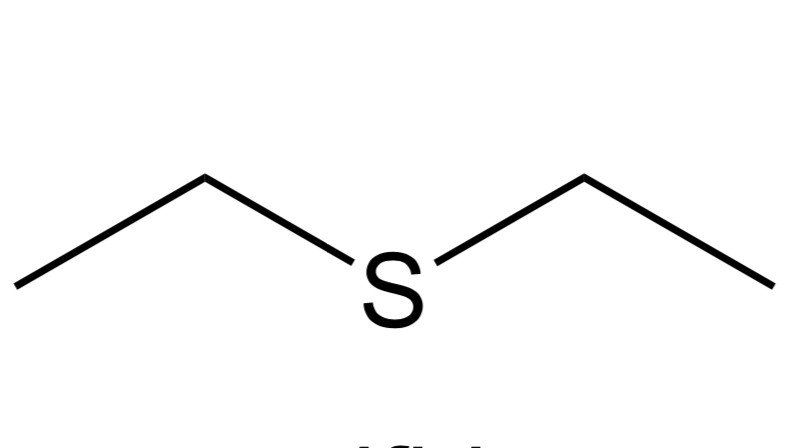
Sulfide
C-S-C group
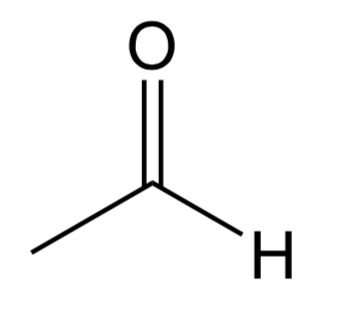
Aldehyde
Has a carbonyl group C=O with a hydrogen. Other bond will be carbon unless it is formaldehyde in which it will be another hydrogen.
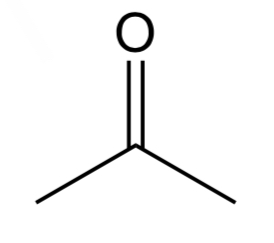
Ketone
Has c =o bond W 2 other carbons attached to the Carbon
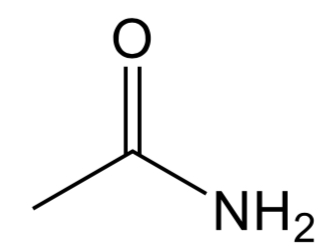
Amide
Has C =o group with a N and C attached to the C in the double bond
Carboxylic acid
With a C=O bond a OH and C to the C.
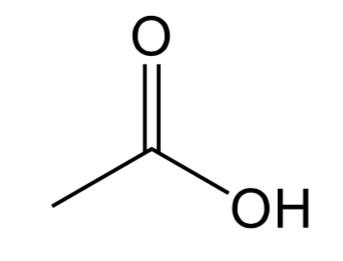
Ester
A C=O bond with a O-C bond and C bonded to the C.
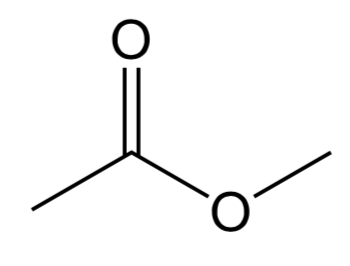
Anhydride
Contains an O atom that bonds two C=O bonds
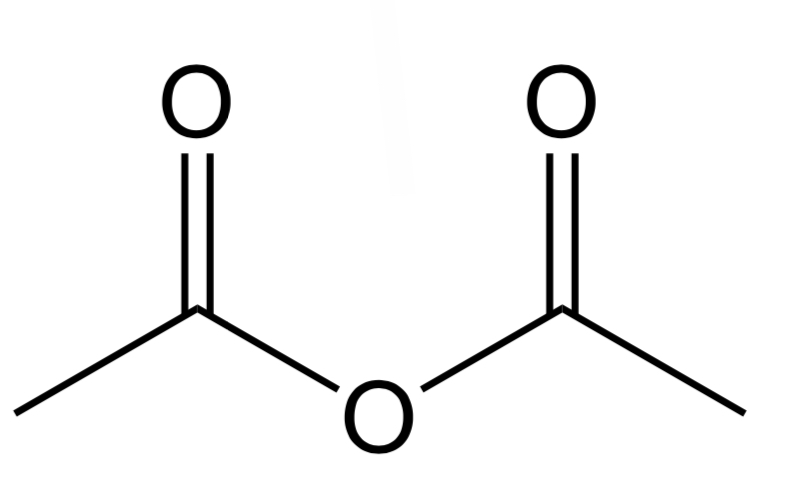
Acid Chloride
A Cl and C atom bonded to the C in C=O
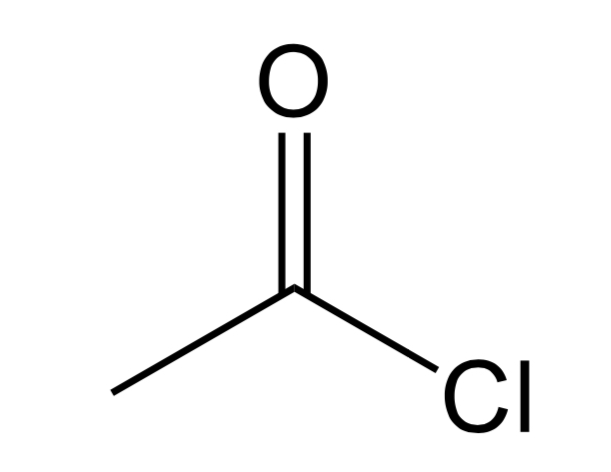
Imine
N=C bond. N will bond with H or C and the C will bond to C or H atoms
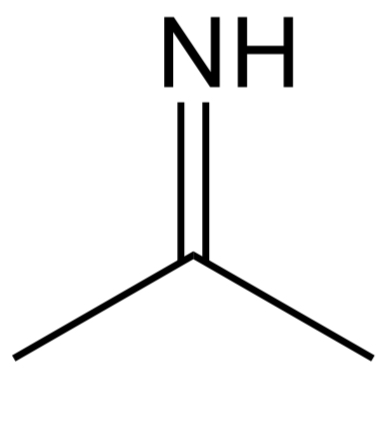
Nitro
Contains a NO2 bond
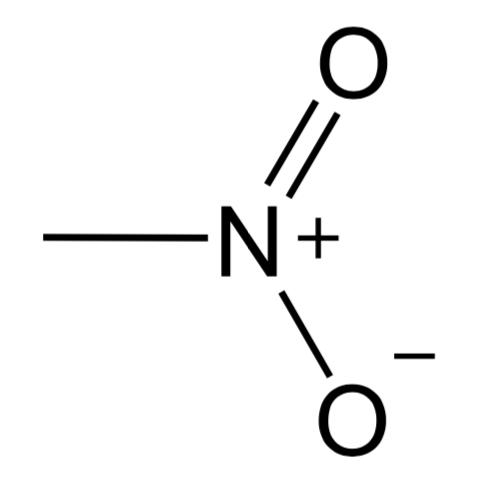
Nitrile
Contains a CN triple bond