Haloalkanes and Haloarenes
1/61
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
Haloalkanes
Replacement of hydrogen with halogen in an aliphatic hydrocarbon results in the formation of alkyl halide (haloalkane)
Haloarenes
Replacement of hydrogen with halogen in an aromatic hydrocarbon results in the formation of aryl halide (haloarene)
Applications of Haloalkanes and Haloarenes
1) Chloramphenicol (chlorine) [from microorganisms] - Treatment of typhoid fever
2) Thyroxine (iodine) [from the body] - Controls growth, deficiency causes goitre
3) Chloroquine (chlorine) - Treatment of malaria
4) Halothane (chlorine+fluorine) - Anaesthetic during surgery
Classification
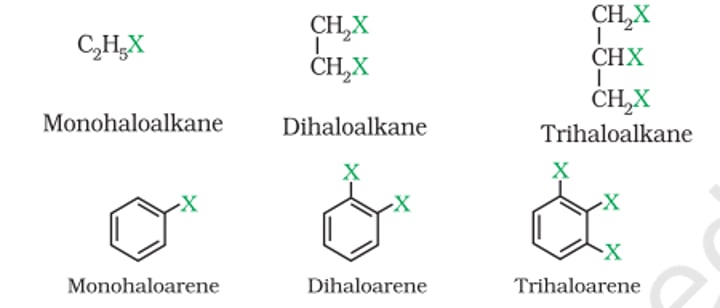
1) Based on the number of Halogen Atoms
2) Based on the molecular structure
3) Based on number of R groups on α Carbon
Based on number of Halogen Atoms
- Monohaloalkane: CH₃-CH₂-Cl
- Dihaloalkane: Cl-CH₂-CH₂-Cl
- Polyhaloalkane: Cl-CH₂-CH(Cl)-CH₂-Cl
- Monohaloarenes: C₆H₅Cl
- Dihaloarenes: C₆H₄Cl₂
- Polyhaloarenes: C₆H₃Cl₃
Based on the Molecular Structure
X is attached to sp³ hybrid carbon:
1) Alkyl Halides
2) Allylic Halides
3) Benzylic Halides
X is attached to sp² hybrid carbon:
4) Vinylic Halides
5) Aryl Halides (or) Haloarenes
Alkyl Halides
Both α and β carbon are single-bonded
CH₃-CH₂-CH₂-X
Allylic Halides
β carbon is double-bonded
CH₂=CH-CH₂-X
Benzylic Halides
β carbon is part of the benzene ring
C₆H₆-CH₂-X
Vinylic Halides
α carbon is double-bonded
CH₂=CH-X
Anyl Halides
X attached to benzene ring
C₆H₅X
Based on number of R groups on α Carbon
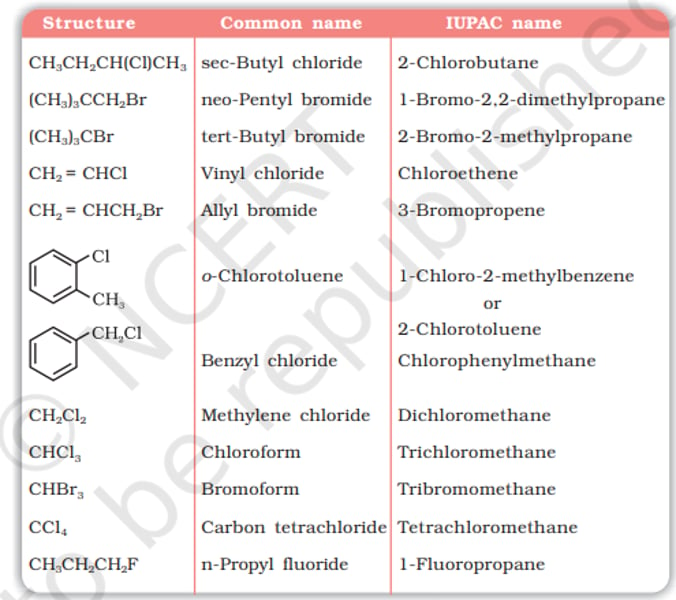
Alkyl Halides:
- Primary (1⁰) [1 β carbon] R-CH₂-X
- Secondary (2⁰) [2 β carbons] CH(R)₂-X
- Tertiary (3⁰) [3 β carbons] CH(R)₃-X
![<p>Alkyl Halides:<br>- Primary (1⁰) [1 β carbon] R-CH₂-X<br>- Secondary (2⁰) [2 β carbons] CH(R)₂-X<br>- Tertiary (3⁰) [3 β carbons] CH(R)₃-X</p>](https://knowt-user-attachments.s3.amazonaws.com/71b0873f-928e-401c-be16-81199ca9acdc.jpg)
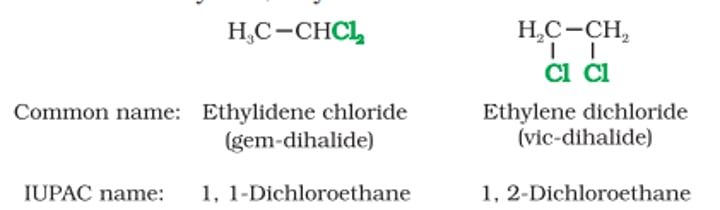
Based on Carbons to which X is attached to dihalides
Dilhalides can be classified into:
1) Geminal Dihalides (gem-dihalides):
Two halogens attached to one carbon
CH₃-CH(Cl)₂
2) Vicinyl Dihalides (vic-dihalides):
Two halogens attached to neighbouring carbons
CL-CH₂-CH₂-Cl
Common IUPAC Names
Nature of C-X Bond
- Halogen atoms are more electronegative
- So, carbon-halogen bond of alkyl halide is polarized
- Carbon gets partial positive charge
- Halogen atom gets partial negative charge
- Then splits into carbocation (C⁺) + nucleophile (X⁻)
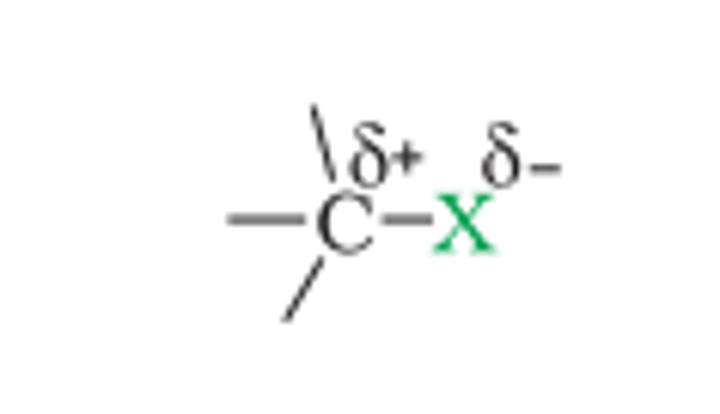
Nomenclature
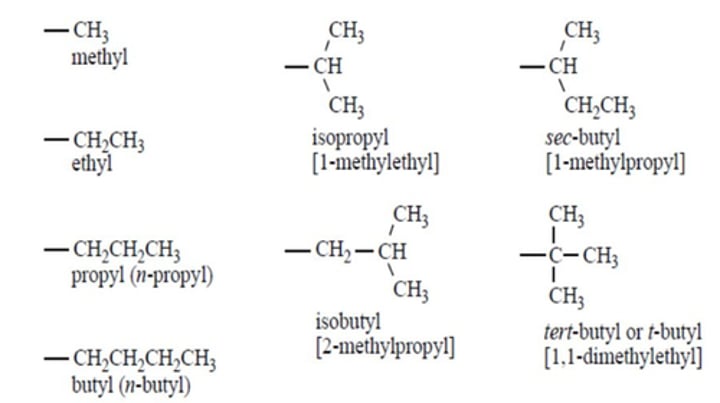
Learn from note
image contains common alkyl group names
Preparation of Haloalkanes
1) From Alcohols
2) From Alkanes
3) From Alkenes
4) Halogen Exchange Reaction
1) From Alcohols
The order of reactivity of alcohols with a given haloacid is 3°>2°>1°
1) R-OH + HCl ->[ZnCl₂] R-Cl + H₂O
ZnCl₂ + con.HCl - Lucas Reagent
2) R-OH + NaBr + H₂SO₄ -> R-Br + NaHSO₄ + H₂O
3) 3R-OH + PX₃ -> 3R-X + H₃PO₃ (X=Cl/Br)
4) R-OH + PCl₅ -> R-Cl + POCl₃ + HCl
5) R-OH + SOCl₂ -> R-Cl + SO₂ + HCl
Thionyl chloride (SOCl₂) is preferred in industries because in this reaction alkyl halide is formed along with escapable gases SO₂ and HCl. So pure alkyl halides are obtained.
The above methods are not applicable for the preparation of aryl halides because the carbon-oxygen bond in phenols has a partial double bond character (due to resonance) and is difficult to break
2) From Alkanes by Free Radical Halogenation
R-H -> [X₂+Energy] R-X+HX
1) CH₄ + Cl₂ -> [Sunlight] CH₃Cl + HCl
2) CH₃-CH₃+Br₂ -> CH₃-CH₂-Br + HBr
3) CH₃-CH₂-CH₃+Cl₂ -> [Sunlight] CH₃-CH(Cl)-CH₃ (major product) + CH₃-CH₂-CH₂-Cl (minor product) + HCl
4) CH₄+ Cl₂ -> [Sunlight] CH₃Cl (monochlorination) -> [Sunlight+Cl₂] CH₂Cl₂ (dichlorination)-> [Sunlight+Cl₂] CHCl₃ (trichlorination) -> [Sunlight+Cl₂] CCl₄
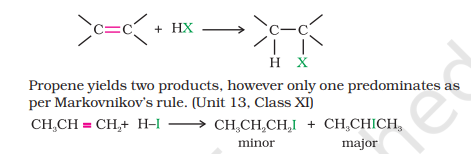
3(i) From Alkenes by Addition of Hydrogen Halides
Follows Markonikov’s Rule (halogen attaches to the most substituted carbon/carbon with the least hydrogens
CH₃-C(CH₃)=CH₂+HBr → CH₃-(Br)C(CH₃)-CH₃
CH₃-C(CH₃)=CH₂+HBr → (peroxide) CH₃-CH(CH₃)-CH₂-Br
C₆H₁₀ (cyclohexane) + HI → C₆H₉I
3(ii) From Alkenes by Addition of Halogens
C=C + X₂ → (dark) X-C-C-X
1)
CH₃-CH=CH₂ + Cl₂ → (sunlight) Cl-CH₂-CH=CH₂ + HCl [undergoes substitution]
CH₃-CH=CH₂ + Cl₂ → (dark) CH₃-CH(Cl)-CH₂(Cl) [undergoes addition]
2)
CH₃-CH₂-CH=CH₂ + Br₂ → (sunlight) CH₃-CH(Br)-CH=CH₂ [undergoes substitution]
CH₃-CH₂-CH=CH₂ + Br₂ → (dark) CH₃-CH₂-CH(Br)-CH₂(Br)
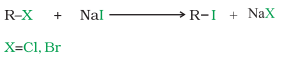
4) Halogen Exchange Reaction
(i) Finkelstein Reaction
R-X + NaI → (dry acetone) R-I +NaX (X=Cl/Br)
NaCl or NaBr formed is precipitated in dry acetone and alkyl iodides are obtained
ii) Swarts Reaction
R-X +AgF → R-F +AgX (X=Cl/Br
any heavy metal fluoride will work
Preparation of Haloarenes
1) From Electrophilic Substitution
2) Sandmeyer’s Reaction
1) From Electrophilic Substitution
Halogenation of benzene (or) benzene derivative
Every electrophilic substitution requires a lewis acid
1) benzene + Cl₂ → [anhydrous AlCl₃] chlorobenzene + HCl
2) (image)
para - major product
ortho - minor product
3) Nitro benzene + Br₂ → [FeBr₃] m-bromo nitro benzene
If the functional group is ortho/para director, the halogen attaches in ortho/para positions
If the functional group is meta director (next flashcard), the halogen attaches in the meta position
![<p>Halogenation of benzene (or) benzene derivative</p><p>Every electrophilic substitution requires a lewis acid</p><p></p><p>1) benzene + Cl₂ → [anhydrous AlCl₃] chlorobenzene + HCl</p><p>2) (image)</p><p>para - major product</p><p>ortho - minor product</p><p></p><p>3) Nitro benzene + Br₂ → [FeBr₃] m-<span>bromo nitro benzene</span></p><p></p><p>If the functional group is ortho/para director, the halogen attaches in ortho/para positions</p><p>If the functional group is meta director (next flashcard), the halogen attaches in the meta position</p>](https://knowt-user-attachments.s3.amazonaws.com/fe5d831e-6db4-42e8-aa6c-d4f5351ffb4d.png)
Meta Directors
-CN (Cyanide)
-NO₂ (Nitro)
-CHO (Aldehyde)
-SO₃H (Sulfonic Acid)
-COOH (Carboxylic Acid)
Any other than these assume as ortho/para director
Mnemonic to remember:
"Cats Never Choose Sour Apples"
Cats for Cyanide (-CN)
Never for Nitro (-NO₂)
Choose for Cho (Aldehyde, -CHO)
Sour for Sulfonic acid (-SO₃H)
Apples for Acid (Carboxylic acid, -COOH)
2) Sandmeyer’s Reaction (from amines)
Sandmeyer’s Reaction:
N₂Cl benzene → [Cu₂Cl₂] Chlorobenzene
N₂Cl benzene → [Cu₂Br₂] Bromobenzene
N₂Cl benzene → [KI] Iodobenzene
N₂Cl benzene → [KCN] Cyano benzene
Gatterman’s Reaction:
N₂Cl benzene → [Cu+HCl] Chlorobenzene
N₂Cl benzene → [Cu+HBr] Bromobenzene
(benzene diazonium chloride)
![<p>Sandmeyer’s Reaction:</p><ol><li><p>N₂Cl benzene → [Cu₂Cl₂] Chlorobenzene</p></li><li><p>N₂Cl benzene → [Cu₂Br₂] Bromobenzene</p></li></ol><p></p><p>N₂Cl benzene → [KI] Iodobenzene</p><p>N₂Cl benzene → [KCN] Cyano benzene</p><p></p><p>Gatterman’s Reaction:</p><ol><li><p>N₂Cl benzene → [Cu+HCl] Chlorobenzene</p></li><li><p>N₂Cl benzene → [Cu+HBr] Bromobenzene</p></li></ol><p></p><p>(<span>benzene diazonium chloride)</span></p>](https://knowt-user-attachments.s3.amazonaws.com/ae8be443-ef83-45e8-b823-d4fb4fbedf3d.png)
Physical Properties
Alkyl halides are mostly liquids and volatile in nature
Sweet-smelling compounds
Pure compounds are colorless but Iodides and Bromides can be colored on storage
Melting and Boiling point increases with an increase in molecular size due to an increase in Van der Waal’s forces
For isomeric haloalkanes, boiling points decrease with an increase in branching. This is because the increase in branching decreases surface area which in turn decreases Van der Waal’s forces
B.P Order - ortho>para>meta (nearly same)
M.P Order - para>ortho>meta
para is highest due to the symmetry of para isomers
When compared with hydrocarbons, haloalkanes have a higher boiling point due to dipole-dipole interactions
The density of haloalkanes increases with an increase in molecular mass. The order of densities of haloalkanes is:
R-F < R-Cl < R-Br < R-I
The solubility of haloalkanes decreases with an increase in size (reason in next flashcard)
Alkyl halides are polar but are immiscible in water. Why?
To dissolve haloalkanes in water, energy is required to overcome the attractions between the haloalkane molecules and the hydrogen bonds in water
But less energy is released when new attractions are set up between haloalkane and water molecules which is not enough to break the hydrogen bonding in water
But haloalkanes are soluble in organic solvents
Chemical Reactions of Haloalkanes
Nucleophilic substitution
Elimination reactions
Reaction with metals
Nucleophilic Substitution
R-X +Nu⁻ → R-Nu + X⁻
R-X + A-B → R-B + AX
R-X + aq.NaOH (or) aq.KOH → R-OH
R-X + KCN → R-CN
R-X + AgCN → R-NC
R-X + KNO₂ → R-ONO
R-X + AgNO₂ → R-NO₂
R-X + LiAlH₄ → R-H
R-X + NH₃ → R-NH₂
R-X + R’-NH₂ → R-NH-R’
R-X + Mg → R-MgX + H₂O → R-H
R-X + NaOR’ → R-O-R’
Ambident Nucleophiles
Nucleophiles with two donor atoms
Example:
-CN and -NC
(nitrite) and (iso cyanide) or (iso nitrite)
NO₂ and ONO
(nitro) and (nitroso)
Mechanism of Nucleophilic Substitution
This reaction has been found to proceed by two different mechanisms:
Substitution Nucleophilic Bimolecular (SN2)
One-step process
Fast process
Removal of halogen and attack of the nucleophile occurs simultaneously
Follows second-order kinetics
Predominantly takes place in primary (1⁰) alkyl halides due to lesser steric hindrance
Results in an inversion of configuration
Substitution Nucleophilic Unimolecular (SN1)
Two-step process
Slow process
Removal of halogen and attack of the nucleophile occurs in a sequence
Follows first-order kinetics
Predominantly takes place in tertiary (3⁰) alkyl halides due to higher stability of 3⁰ carbocations (3⁰C⁺>2⁰C⁺>1⁰C⁺)
Results in racemisation
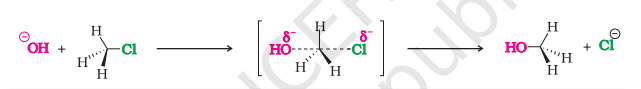
Substitution Nucleophilic Bimolecular (SN2) Mechanism
The nucleophile cannot approach in the same direction as X because both are electron-rich, so it attacks from behind
Both nucleophile and halogen will be attached (transition state) and the compound becomes unstable
The halogen gets removed as X⁻
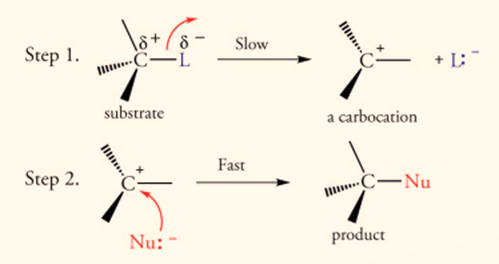
Substitution Nucleophilic Unimolecular (SN1) Mechanism
Step 1: Removal of Halogen
Step 2: Attack of Nucleophile
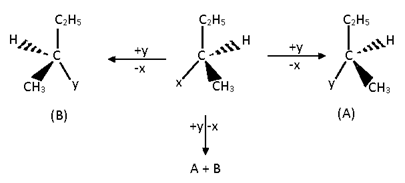
there’s a 50% chance of the image product forming and a 50% chance of the mirror image of that forming
Inversion Retention and Racemisation
SN1 has a 50% chance of retention and a 50% chance of inversion
SN2 has a 100% chance of inversion
Order of Reactivity towards SN1 and SN2 Mechanism
SN2 Mechanism Increases——————————————————→
Benzyl Halides (most steric hindrance/most stable C⁺); Allyl Halides; 3⁰ Halides; 2⁰ Halides; 1⁰ Halides; Methyl Halides (least steric hindrance/least stable C⁺)
←—————————————————SN1 Mechanism Increases
More stable C⁺ = More reactive SN1
Less steric hindrance = More reactive SN2
Stereoscopic Aspects of Nucleophilic Substitution
Optically Active Compounds
Optical Isomers
Optically Active Compounds
Compounds which can rotate plane polarised light passing through it are called optically active compounds
Opitcal Isomers
Two compounds with the same molecular formula but differ in the direction of rotation of plane polarised light are called optical isomers
Two types:
Dextro Rotatory (D-Isomer): Clockwise Rotating (+)
Laevo Rotatory (L-Isomer): Anti-clockwise Rotating (-)
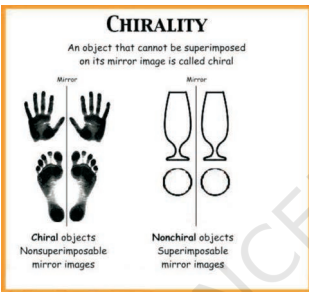
Chirality
An object that cannot be superimposed on its mirror image is called chiral
A compound will be optically active if it has a chiral carbon
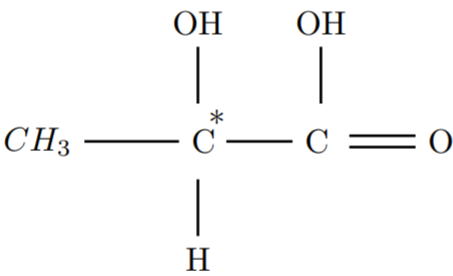
A chiral carbon will have 4 different function groups attached to it
Chiral Carbon Example
A chiral carbon will have 4 different function groups attached to it
Enantiomers
Two optically active compounds which can form non-superimposable mirror images of each other are collectively called enantiomers
In a pair of enantiomers, one compound is an optical isomer of the other
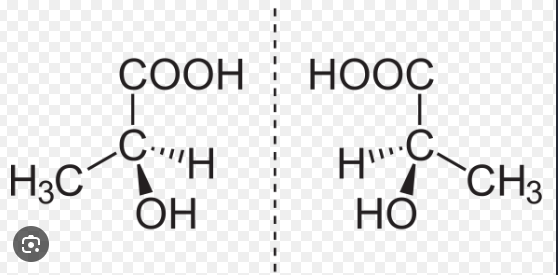
Racemisation
An equimolar mixture of dextro and laevo rotatory isomers is called a racemic mixture
The process of preparing a racemic mixture is known as racemisation
The net optical activity of a racemic mixture is zero because the clockwise rotation of D-Isomer (+) is cancelled by the anti-clockwise rotation of the L-Isomer (-)\
Ex:
(+/-) Butan-2-ol
(+) Butal-2-ol: D-isomer; (-) Butal-2-ol: L-isomer, (+/-) Butan-2-ol: Racemix Mixture (50%/50%)
(+/-) 2-Chloro Butane
Nucleophilic Substitution in Haloarenes
Aryl halides are extremely less reactive towards nucleophilic substitution reaction due to:
Resonance Effect: The C-X bond acquires a partial double bond character due to resonance which is difficult to break
X attached to an sp² hybrid carbon: This is a stronger bond (more %s character) than the bond in haloalkane (to an sp³ carbon) so it is harder to break
Instability of phenyl cation: The phenyl cation formed as a result of self-ionisation will not be stabilised by resonance
Repulsion: It is less likely for the electron-rich nucleophile to approach electron-rich arenes and be repelled since a benzene ring is always electron-rich
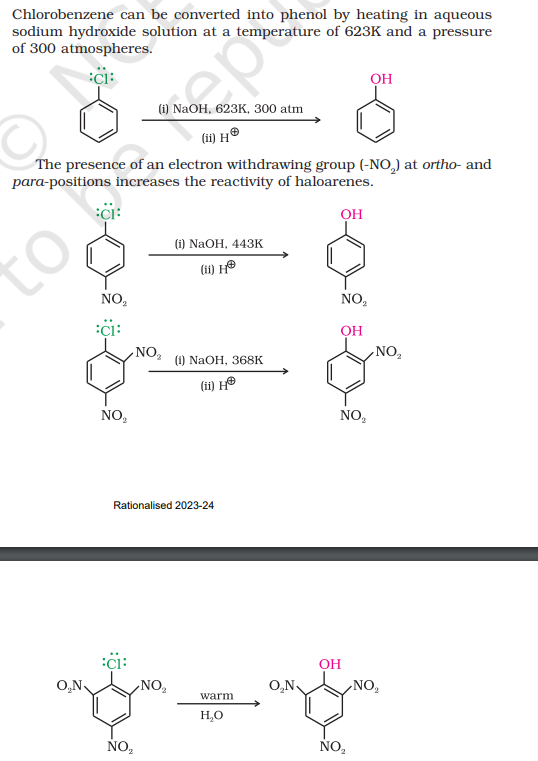
Replacement by hydroxyl group
To make the haloarene more reactive towards a nucleophile, more energy can be supplied to break the double bond or an electron-withdrawing group (-NO2 ) at ortho- and para-positions to weaken the C-Cl bond
First reaction (image) - Dow’s Process
The greater the number of nitro groups - the reactivity of the halo arene increases and less energy is required
Elimination Reaction (or) β-Elimination (or) Dehydrohalogenation
Alexander’s Rule - In dehydrohalogenation reactions, the preferred product is the alkene which has a greater number of alkyl groups attached to the doubly bonded carbon atoms
X-C-C-H → [alcoholic KOH] C=C + HX
CH₃-CH₂-CH₂-Br₂ → [alcoholic KOH] CH₃-CH=CH₂ + HBr
CH₃-CH(Cl)-CH₃ → [KOH + ethano] CH₃-CH=CH₂
CH₃-CH(Cl)-CH₂-CH₃ → [KOH + propanol] CH₃-CH=CH-CH₃
CH₃-C(CH₃)(Br)-CH₂-CH₃ → [alcoholic KOH] CH₃-C(CH₃)=CH-CH₃
Reaction with Metals
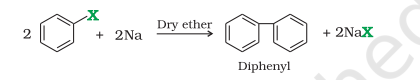
1) Wurtz Reaction
2) Fittig Reaction
3) Wurtz-Fittig Reaction
4) Preparation of Grignard Reagent
Wurtz Reaction
R-X → (Na + Dry Ether) R-R + NaX
CH₃-CH₂-Br → (Na + Dry Ether) CH₃-CH₂-CH₂-CH₃ +NaBr
CH₃-CH(CH₃)-Cl → (Na + Dry Ether) CH₃-CH(CH₃)-CH(CH₃)-CH₃ + NaCl
Fittig Reaction
don’t have to balance reaction
Wurtz-Fittig Reaction
don’t have to balance reaction
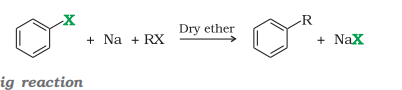
Preparation of Grignard Reagent
R-X + Mg → [dry ether] R-MgX
CH₃-Br + Mg → [dry ether] CH₃-MgBr
bromobenzene/chlorobenzene + Mg → [dry ether] cyclo hexyl magnesium chloride/bromide
Dry ether is used because if there was a presence of moisture Grignard reagent would react with water and forms a hydrocarbon
R-MgX + H₂O → RH + OH-Mg-X
Electrophilic Substitution Reactions for Haloarenes
Halogenation
Nitration
Sulphonation
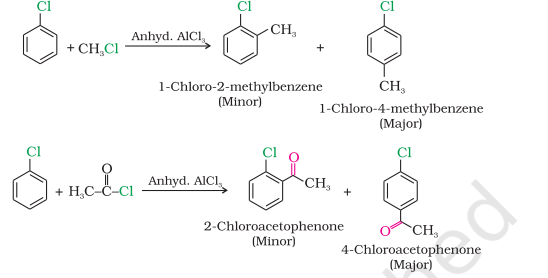
Friedel-Crafts reaction
Halogenation
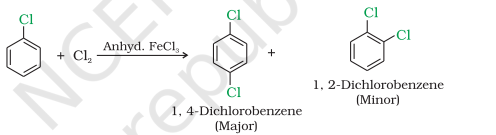
Nitration
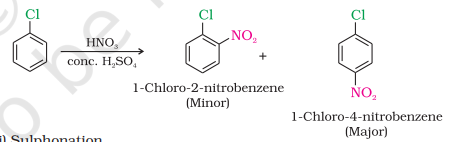
Sulphonation
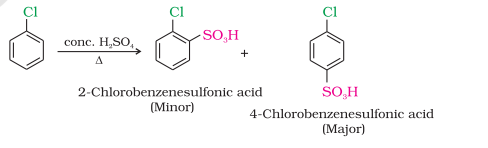
Friedel-Crafts reaction
Polyhalogen Compounds
Dichloromethane (Methylene chloride)
Trichloromethane (Chloroform)
Triiodomethane (Iodoform)
Tetrachloromethane (Carbon tetrachloride)
Dichloromethane
Trichloromethane
Triiodomethane
Tetrachloromethane