8.4 Transport of O2 & CO2 in blood
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Erythrocytes adaptations
Have biconcave shape
Gives large SA for gaseous exchange
Helps them pass thru narrow capillaries
Contain O2 carrying pigment haemoglobin
Mature ones have no nucleus
More room for maximum amount of haemoglobin
In cytoplasm contains enzyme carbonic anhydrase involved in carriage of CO2 in blood
How long can RBCs last?
120 days
Where are erythrocytes fomed?
Red bone marrow continuously
Haemoglobin
Red pigement that carries oxygen
Gives erythrocytes their color
Water soluble
Large globular protein
Made of 4 peptide chains, each w. an iron-containing haem prosthetic group
2 alpha subunits, 2 beta subunits
300 million molecules in each RBC
How many oxygens can bind to 1 haemoglobin molecule
4
Each haem binds to 1 O2
Oxygen binding to haemoglobin
Oxygen binds quite loosely to haemoglobin forming oxyhaemoglobin
Reaction is reversible

How does oxygen fit onto haemoglobin?
The arrangement of the haemoglobin molecule means that as soon as 1 O2 molecule binds to a haem group, the molecule changes shape,
Makes it easier for the next O2 molecules to bind
How is oxygen carried from lungs?
When erythrocytes enter capillaries in lungs, O2 levels in cells are relatively low
Makes steep conc. gradient between inside of erythrocytes & air in alveoli
O2 moves into erythrocytes & binds w. haemoglobin
As O2 is bound to haemoglobin, free O2 conc. in erythrocyte stays low
Maintains steep diffusion gradient until haemoglobin completely saturated w. O2
Lungs and Oxygen
High pO2
Haemoglobin in RBCs is rapidly loaded w. O2
10-12kPa
High affinity
High conc. of O2
When blood reached body tissues, how is O2 let off?
Conc. of O2 in cytoplasm of body cells is lower than in erythrocytes
O2 moves out of erythrocytes down a conc. gradient
Once 1st O2 molecule is released by the haemoglobin, the molecule changes shape
Makes it easier to remove remaining O2 molecules
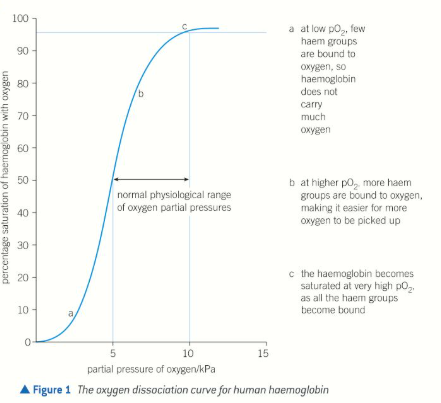
Oxygen dissociation curve
Shows affinity of haemoglobin for O2
Curve levels out at the highest partial pressures of O2
All haem groups are bound to O2
Haemoglobin is saturated & cannot take up any more
Respiring tissues & O2
Low pO2
2-4kPa
Low affinity (drops off O2)
A relatively small drop in O2 levels in respiring tissues means O2 is released rapidly from haemoglobin to diffuse into the cells
Effect is enhanced by relatively low pH in tissues compared w. lungs
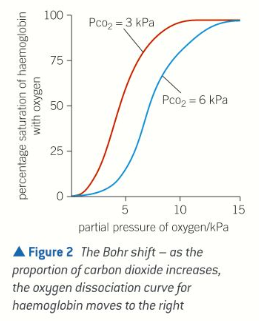
Low pO2
pCO2 is high
Occurs in respiring tissues
Haemoglobin has low affinity at low pO2 in order to drop off O2 at respiring tissues
Bohr effect
At higher partial pressures of CO2, haemoglobin gives up O2 more easily
Why is the Bohr effect important in the body?
In active tissues w. a high partial pressure of CO2, haemoglobin gives up its O2 more readily
In lungs where proportion of CO2 in air is relatively low, O2 bind to haemoglobin molecules easily
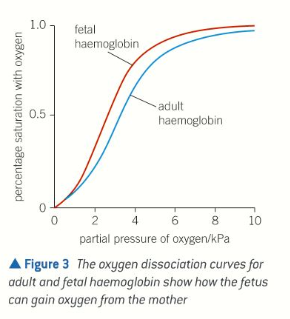
Foetal haemoglobin
Has higher affinity for O2 than maternal haemoglobin
Oxygenated blood from mum runs close to deoxygenated blood in placenta
If blood of fetus had same affinity for O2 as blood of mum, little or no O2 would be transferred to blood of fetus
Having higher affinity allows fetal haemoglobin to remove O2 from maternal blood as the bloods run past each other
What is fetal haemoglobin made from?
2 alpha
2 gamma
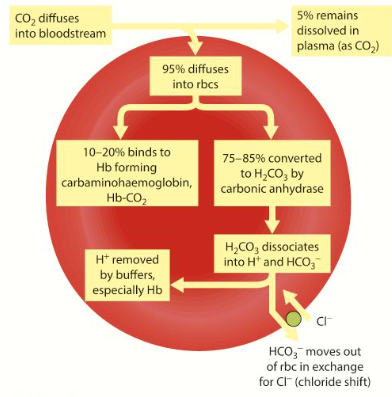
3 ways in which CO2 is transported from tissues to lungs
About 5% is carried dissolved in plasma
10-20% is combined w. amino groups in polypeptide chains of haemoglobin to form carbaminohaemoglobin
75-85% is converted into hydrogen carbonate ions in the cytoplasm of RBCs
CO2 + H2O
In blood plasma CO2 reacts slowly w. H20 to form carbonic acid
Carbonic acid then dissociated to form H+ ions & hydrogen carbonate ions (HCO3-)
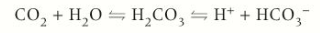
Why is the reaction between CO2 & H2O faster in cytoplasm of RBCs?
Due to high levels of carbonic anhydrase enzyme
Catalyzes reversible reaction
Chloride Shift
Negatively charged HCO3- ions move out of erythrocytes into plasma by difffusion, down conc. gradient
Negatively charge chloride ions (Cl-) move into erythrocytes
What is the point of the chloride shift?
Maintains electrical balance of the cell
Reason for removing CO2 & converting into HCO3- ions
Erythrocytes maintain a steep conc. gradient for CO2 to diffuse from respiring tissues into erythrocytes.
What does carbonic anhydrase do when blood reaches lung tissue?
Here, there is a relatively low conc. of CO2
Carbonic anhydrase catalyzes reverse reaction
Breaks down carbonic acid into CO2 + H2O
HCO3- ions diffuse back into erythrocytes & react with H_ ions to form more carbonic acid
What happens when more carbonic acid is broken down by carbonic anhydrase
It releases free CO2
This diffuses out of blood into lungs
Chloride ions diffuse out of RBCs back into plasma down an electrochemical gradient
How does haemoglobin play a role in transporting CO2?
Acts as a buffer
Prevents changes in pH by accepting free H+ ions in a reversible reaction to form haemoglobinic acid

Myoglobin is an O2 binding molecule found in muscles. From the graph, explain the differences between the 2 curves.
Myoglobin in muscles has higher oxygen affinity than haemoglobin in blood
So muscles can take oxygen from haemoglobin in blood
Enables muscles to get extra oxygen when they are contracting during exercise