Kaplan MCAT Organic Chemistry chapter 4: Analyzing Organic Reactions
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Lewis Acid
electron acceptor in the formation of a covalent bond
-tend to be electrophiles
-have vacant p-orbitals which can accept an electron pair, or are positively polarized atoms
Lewis Base
electron donor in the formation of a covalent bond
-tend to be nucleophiles
-have a lone pair of electrons that can be donated and are often anions
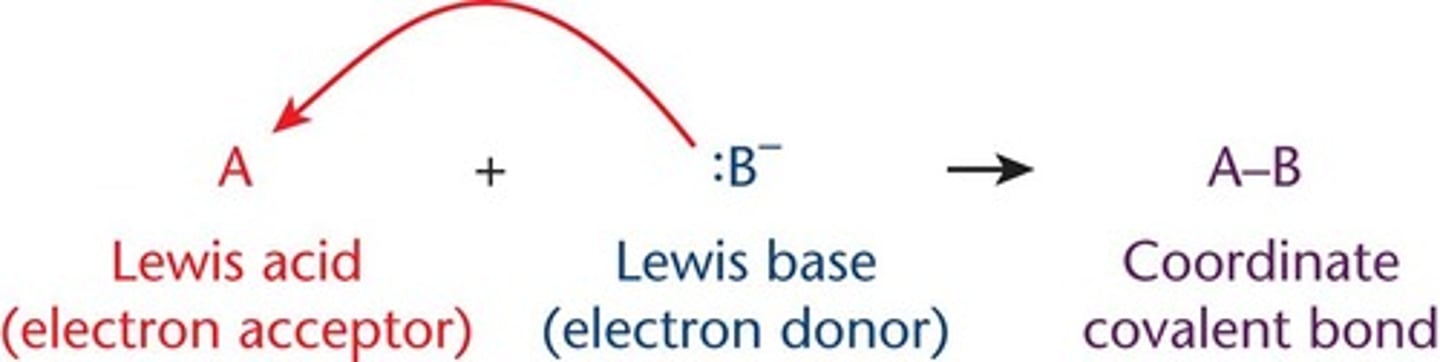
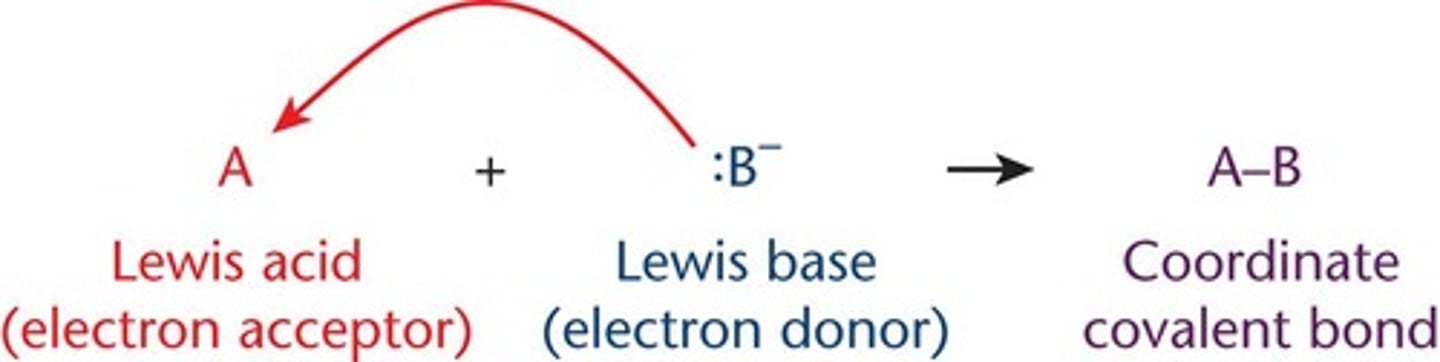
coordinate covalent bonds
form when lewis acids and bases interact
-electrons in the bond come from the same starting atom (the Lewis base)
Bronsted-Lowry acid
proton donor
amphoteric
ability to act as either Bronsted-Lowry acid or base (e.g. water, Al(OH)3, HCO3−, and HSO4−)
Al(OH)3
aluminum hydroxide,
acts as base:
3 HCl + Al(OH)3 → AlCl3 + 3 H2O
acts as acid:
Al(OH)3 + OH− → Al(OH)4−
Bronsted-Lowry base
proton acceptor
acid dissociation constant (ka)
measures the strength of an acid in solution
Ka equation
In the dissociation of an acid HA (HA ⇋ H+ + A-), the equilibrium constant is given by:
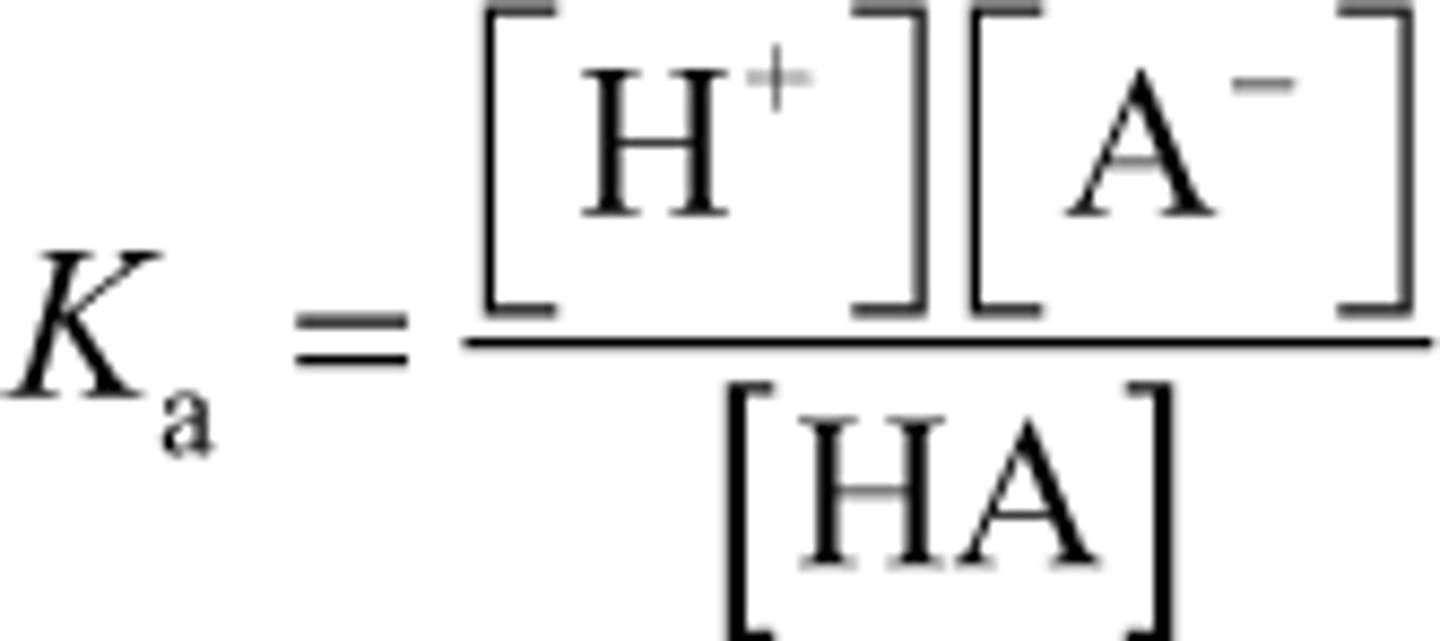
pKa
-logKa
-the lower the pKa the stronger the acid
-some pKa can be negative
strong acid
pKa below -2
weak acid
pka between -2 and 20
pKa values for common functional groups
acidity increases with decreasing bond strength to H.
The more electronegative the atom, the higher the acidity
When the above two trends oppose each other, low bond strength takes precedence.
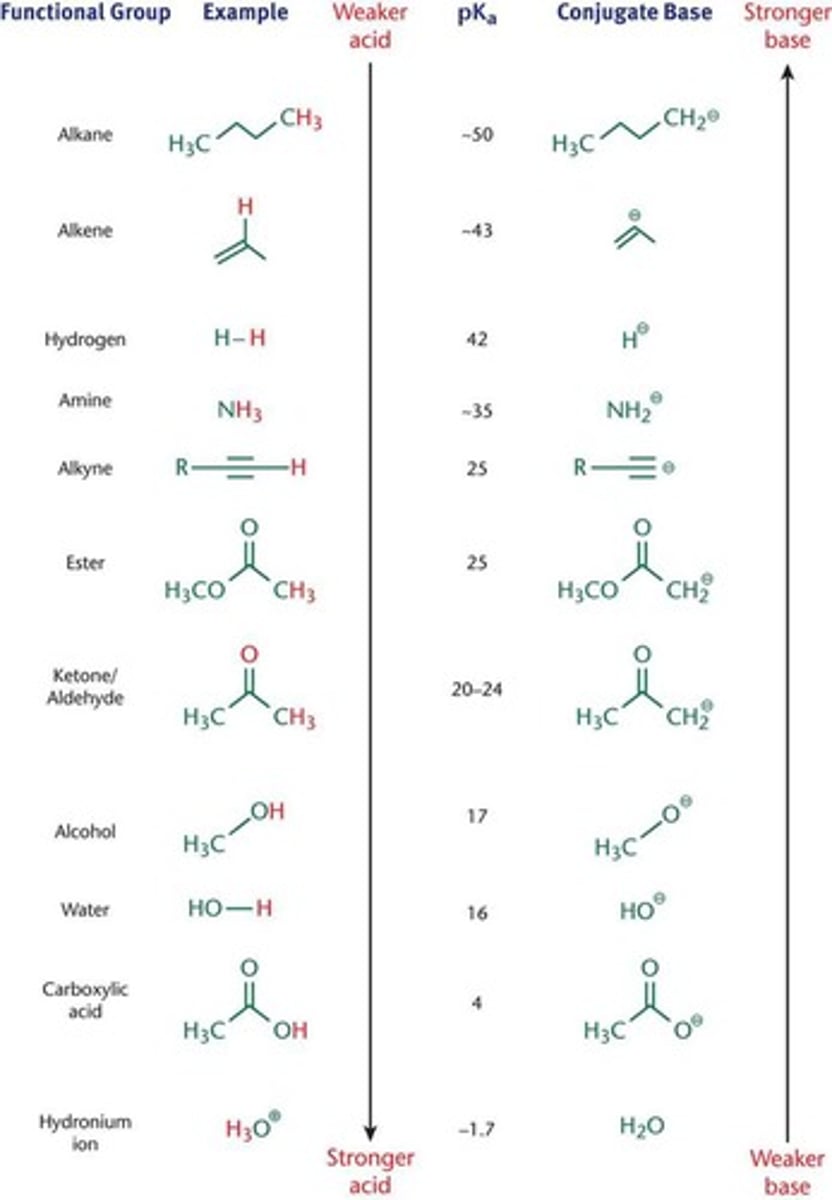
functional groups that act like acids:
alcohols, aldehydes, ketones (at the lphaa-carbon), carboxylic acids, and most carboxylic acid derivatives.
Easier to target basic or nucleophilic reactants because they accept a lone pair
functional groups that act like bases:
amines, amides. N can form coordinate covalent bonds by donating a lone pair to a Lewis acid
Nucleophile
defined as nucleus loving species, with either lone pairs or pi bonds that can form new bonds to electrophiles
Difference between nucleophile strength and base strength
nucleophile strength: is based on the relative rates of reaction with a common electrophile, so it is a kinetic property.
base strength: related to the equilibrium position of the reaction and is therefore a thermodynamic property
good nucleophiles tend to be good bases
the more basic a nucleophile, the more reactive it is
Four factors that determine nucleophilicity
1. charge: nucleophilicity increases with increasing electron density (more negative charge)
2. electronegativity: nucleophilicity decreases as electronegativity increases since these atoms are less likely to share their electron density
3. steric hindrance: bulkier molecules are less nucleophilic
4. solvent: protic (hydrogen attached to an oxygen or nitrogen) solvents can hinder nucleophilicity by protonating the nucleophile or through hydrogen bonding
in polar protic solvents, nucleophilicity ________ down the periodic table
increases
-protons in solution will attract nucleophile, instead of electrophile attracting nucleophile
In order from most nucleophilic to least nucleophilic
I-, Br-, Cl-, F-
in aprotic solvents, nucleophilicity ________ as you go up the periodic table
increases
-no protons get in the way of the attacking nucleophile. in these solutions, the nucleophilicity relates directly to basicity
In order from most nucleophilic to least nucleophilic:
F-, Cl-, Br-, I-
Examples of polar protic and polar aprotic solvents
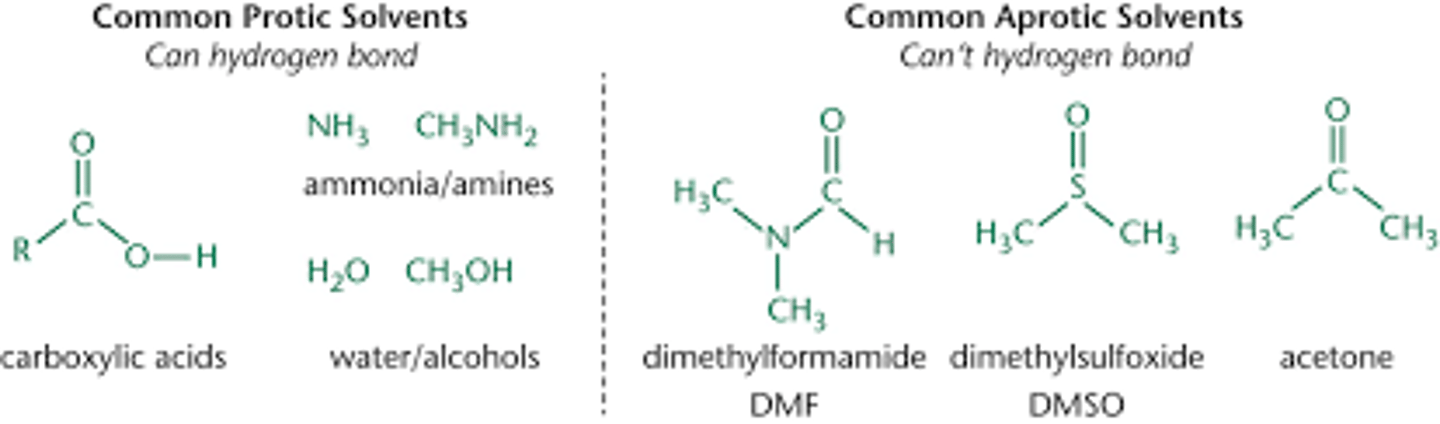
can you use a nonpolar solvent in a nucleophile-electrophile reaction?
no since the reactants are polar. in a nonpolar solvent they would not dissolve
Examples of strong nucleophiles
HO-, RO-, CN-, N3-. Amine groups tend to be good nucleophiles
examples of fair nucleophiles
NH3, RCO2-
examples of weak nucleophiles
H2O, ROH, RCOOH
electrophiles
"Electron-loving" atoms with a positive charge or positively polarized atom that accepts an electron pair when forming bonds with a nucleophile
-almost always act as lewis acid in reaction
-greater degree of positive charge increases electrophilicity: carbocation is more electrophilic than a carbonyl carbon
-if electrophile does not empty orbital, then better leaving groups makes it more likely reaction would occur, leading to greater electrophilicity
-if electrophile contains empty orbital, nucleophile can bond without displacing leaving group
-Ex: alcohols, aldehydes, ketones, carboxylic acids and their derivatives

comparisons of electrophilicity of carboxylic acid derivatives
anhydrides are most reactive, followed by carboxylic acids and esters, then amides
-derivatives of higher reactivity can form derivatives of lower reactivity, but not vice versa
leaving groups
the molecular fragments that retain the electrons after heterolysis, where a bond is broken and both electrons are given to one of the two products.
-the best leaving group will be able to stabilize extra electrons. Resonance and inductive effects from electron withdrawing groups can stabilize negative charge.
-weak bases make good leaving groups (conjugate bases of strong acids, like I-,Br-, Cl-)
-alkanes and hydrogen ions will almost never serve as leaving groups since they form very reactive and strong basic anions
-Another way to think about leaving group: the weaker the base is the leaving group while the stronger base (nucleophile) replaces the weaker base
heterolytic reactions
opposite of coordinate covalent bond formation: a bond is broken and both electrons are given to one of the two products.
Nucleophilic substitution
A type of substitution reaction in which a nucleophile is attracted to an electron-deficient center or atom, where it donates a pair of electrons to form a new covalent bond.
the nucleophile must be more reactive than the leaving group, otherwise leaving group will attach right back.
Unimolecular nucleophilic substitution (SN1) reactions
Step 1: leaving group leaves and is the rate-limiting step. this generates a positively charged carbocation
Step 2: nucleophile attacks the carbocation
the more substituted the carbocation, the more stable it is because the alkyl groups act as electron donors which stabilize the positive charge, so SN1 only occurs for secondary or tertiary carbon
since first step is rate limiting step, the rate of reaction only depends on substrate: rate-k[R-L], where L is leaving group.
-anything that accelerates the formation of the carbocation will increase the rate of SN1 reaction
usually yield racemic mixture since the incoming nucleophile can attack the carbocation from either side
![<p>Step 1: leaving group leaves and is the rate-limiting step. this generates a positively charged carbocation</p><p>Step 2: nucleophile attacks the carbocation</p><p>the more substituted the carbocation, the more stable it is because the alkyl groups act as electron donors which stabilize the positive charge, so SN1 only occurs for secondary or tertiary carbon</p><p>since first step is rate limiting step, the rate of reaction only depends on substrate: rate-k[R-L], where L is leaving group.</p><p>-anything that accelerates the formation of the carbocation will increase the rate of SN1 reaction</p><p>usually yield racemic mixture since the incoming nucleophile can attack the carbocation from either side</p>](https://knowt-user-attachments.s3.amazonaws.com/c7f1e2d7-e5df-4650-abec-bad8514a700e.jpg)
Mechanism of SN1 Reaction
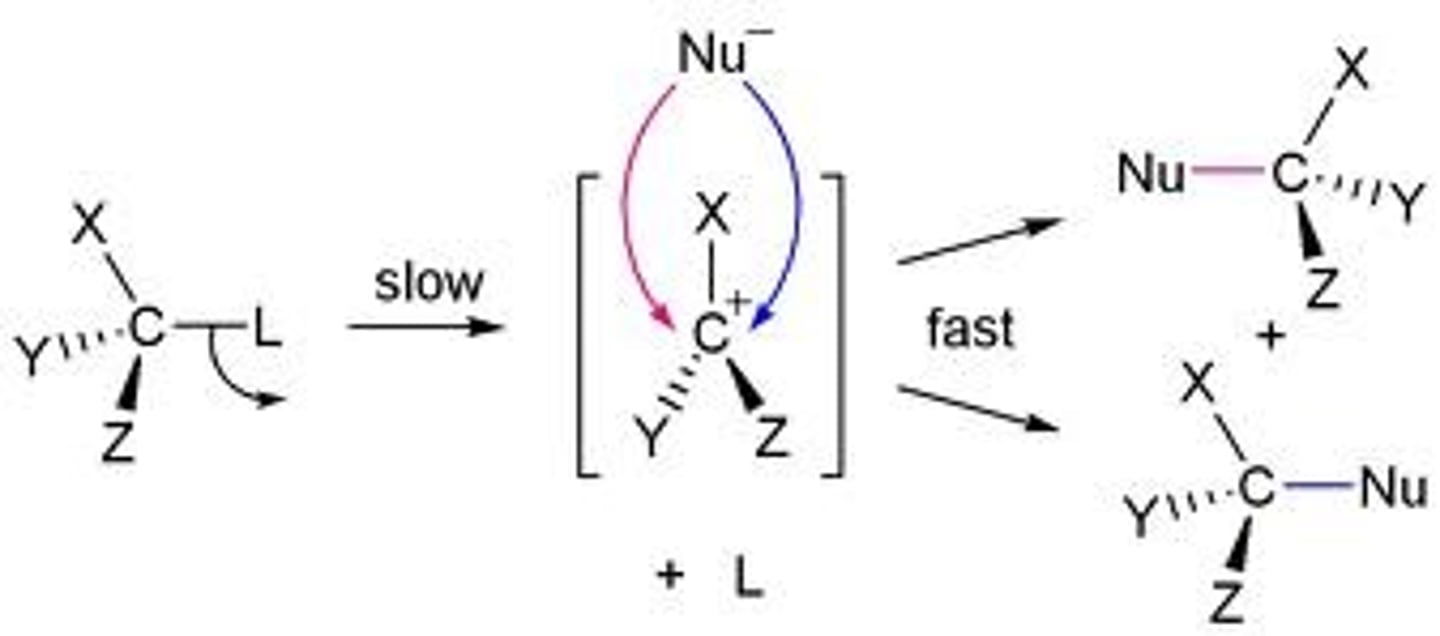
bimolecular nucleophilic substitution (Sn2)
reactions contain only one step (concerted reaction): the nucleophile attacks the compound at the same time as the leaving group leaves'
-cause an inversion of absolute configuration (S and R will switch)
nucleophile actively displaces the leaving group in backside attack
nucleophile must be strong and the substrate cannot be sterically hindered
the less substituted the carbon, the more reactive it is in SN2 reactions
Mechanism of SN2 Reaction
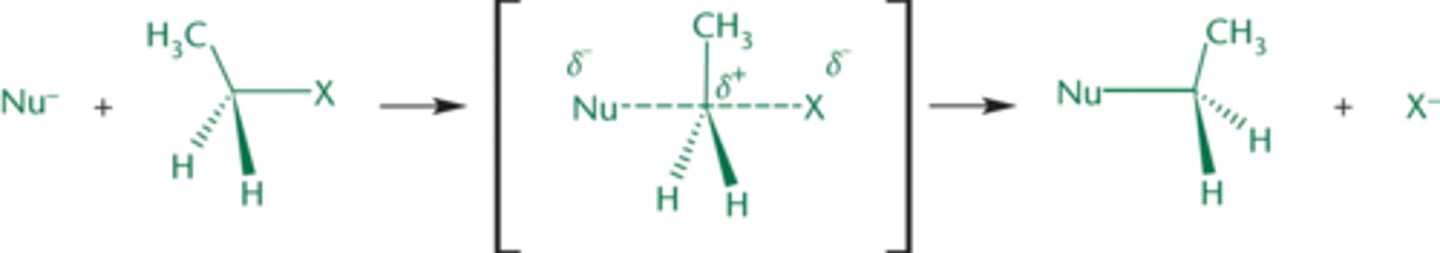
stereospecific reaction
configuration of the reactant determines the configuration of the products due to the reaction mechanism
oxidation-reduction reaction
any chemical change in which one species is oxidized (loses electrons) and another species is reduced (gains electrons)
oxidation state
an indicator of the hypothetical charge that an atom would have if all bonds were completely ionic
CH4 is the most reduced form of carbon, as C has oxidation state of -4.
CO2 is the most oxidized form of carbon, as C has oxidation state of 4
Don't need to know how to assign oxidation states
oxidation
refers to the increase in oxidation state, which means a loss of electrons (easier to view oxidation as increasing the number of bonds to oxygen, other carbons, nitrogen, or halides and removal of bonds to H). Bond between C and a atom less electronegative than C is replaced with bond to an atom more electronegative than C.
reduction
refers to the decrease in oxidation state, which means gaining of electrons (increasing the number of bonds to hydrogen or removal of bonds to O)
functional groups from least to most oxidized
Level 0 (no bonds to heteroatoms): alkanes
Level 1 (1 bond to heteroatoms): alcohols, alkyl halides, amines
Level 2 (2 bonds to heteroatoms): aldehydes, ketones, imines
Level 3 (3 bonds to heteroatoms): carboxylic acids, anhydrides, esters, amides
Level 4 (four bonds to heteroatoms): carbon dioxide
Note that oxidation is in reference to the C atom
oxidizing agent
element or compound in a redox reaction that accepts an electron from another species
-it is reduced
-good oxidizing agents have a high affinity for electrons or high oxidation states ex: O2, O3, Cl2, MnO4-, Mn7+, Cr6+, and CrO4(2-)
Two themes:
1. oxidation reactions tend to feature an increase in the number of bonds to oxygen
2. oxidizing agents often contain metals bonded to a large number of oxygen atoms
Oxidation of alcohols
Primary alcohols can be oxidized by one level to become aldehydes, or can be further oxidized to form carboxylic acids.
-This reaction commonly proceeds all the way to the carboxylic acid when using strong oxidizing agents such as chromium trioxide (CrO3) or sodium or potassium dichromate (Na2Cr2O7 or K2Cr2O7), but can be made to stop at the aldehyde level using specific reagents such as pyridinium chlorochromate (PCC).
secondary alcohols can be oxidized to ketones only
reduction of carbon atom
when a bond between a carbon atom and an atom that is more electronegative than carbon is replaced by a bond to an atom that is less electronegative than carbon.
In practice, this usually means increasing the number of bonds to hydrogen and decreasing the number of bonds to other carbons, nitrogen, oxygen, or halides.
Aldehydes and ketones would be reduced to primary and secondary alcohols.
Amides can be reduced to amines, carboxylic acids can be reduced to alcohols, and esters can be reduced to a pair of alcohols using LiAlH4
Two themes:
1. reduction reactions tend to feature an increase in the number of bonds to hydrogen
2. reducing agents often contain metals bonded to a large number of hydrides
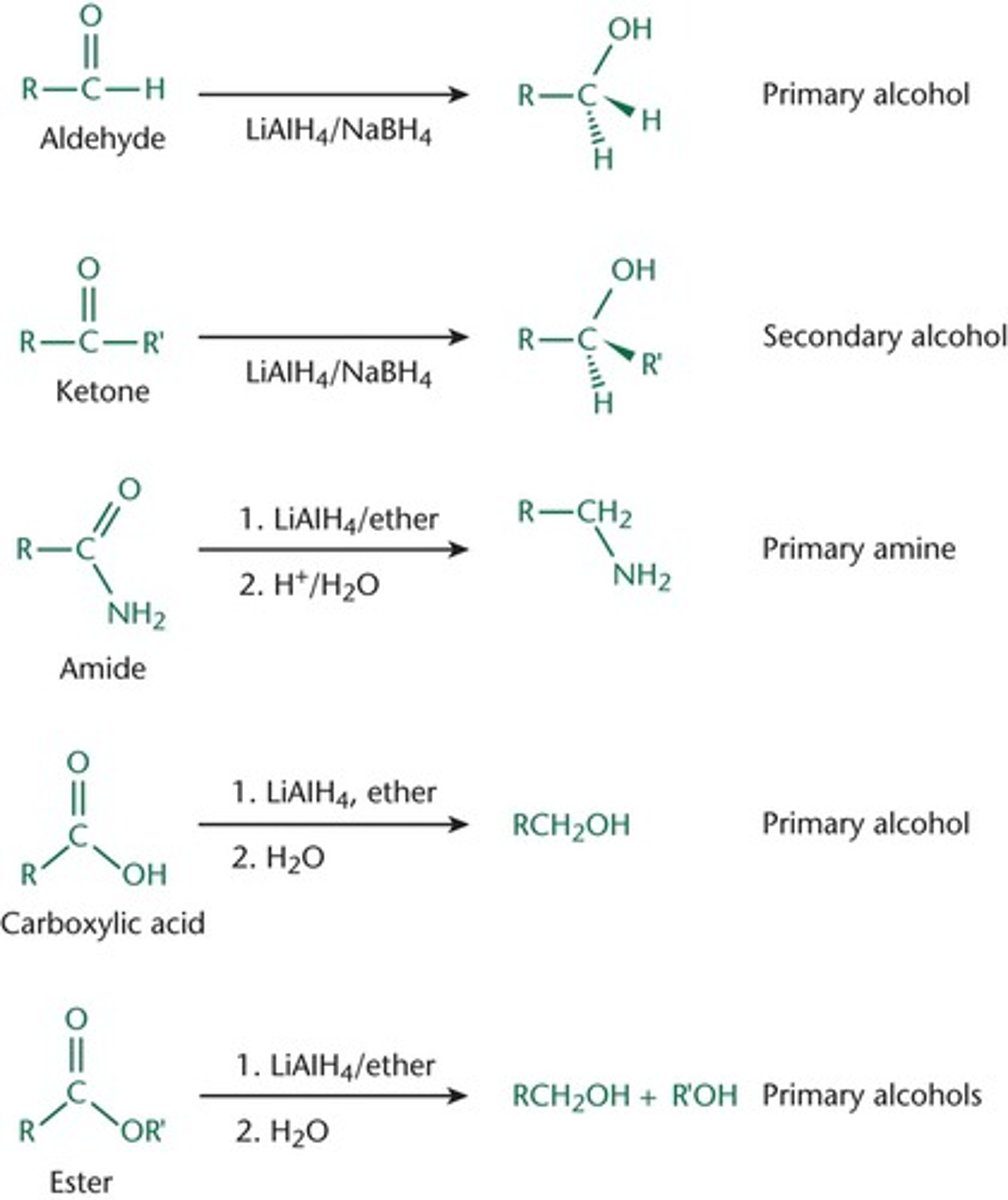
good reducing agents
sodium, magnesium, aluminum, and zinc, metal hydrides (NaH, CaH2, LiAlH4, and NaBH4 since they contain the H- ion).
Chemoselectivity
preferential reaction of one functional group in the presence of another functional group
Nucleophilic-eletrocphilic reactions occur on the highest priority functional group since it usually contains the most oxidized carbon.
Nucleophile is looking for electrophile (positive partial charge on C).
Aldehydes generally more reactive toward nucleophiles than ketones because they are less sterically hindered
common reactive site: the carbon of a carbonyl
-found in carboxylic acid and its derivatives, ketones, and aldehydes
-Carbon acquires a positive polarity due to the electronegativity of the oxygen. Carbonyl carbon becomes electrophilic and can be targeted by nucleophiles
-alpha- Hydrogens are much more acidic than in a regular C-H bond. This occurs due to resonance stabilization of the enol form after H is pulled off. These can be deprotonated easily with a strong base which would form an enolate.
Enolate then becomes a strong nucleophile and alkylation can result if good electrophiles available.
steric hindrance
describes the prevention of reactions at a particular location within a molecule due to the size of substituent groups
e.g. SN2 reaction won't occur with tertiary substrates
steric protection
The Prevention of the formation of alternative products using a protecting group such as a mesylate or tosylate.
protecting group
uses sterics to protect leaving groups.
-a reactive leaving group can be temporarily masked with a sterically bulky group, forcing reactive nucleophile to look elsewhere to react
Steps in Problem Solving
1. know your nomenclature
2. identify the functional groups
3. identify the other reagents
4. identify the most reactive functional group (s): more oxidized C tend be more reactive to both nucleophile-electrophile interactions and redox reactions.
5. identify the first step of the reaction (ex: HOCH2CH2OH is commonly used as protecting group)
6. consider stereoselectivity