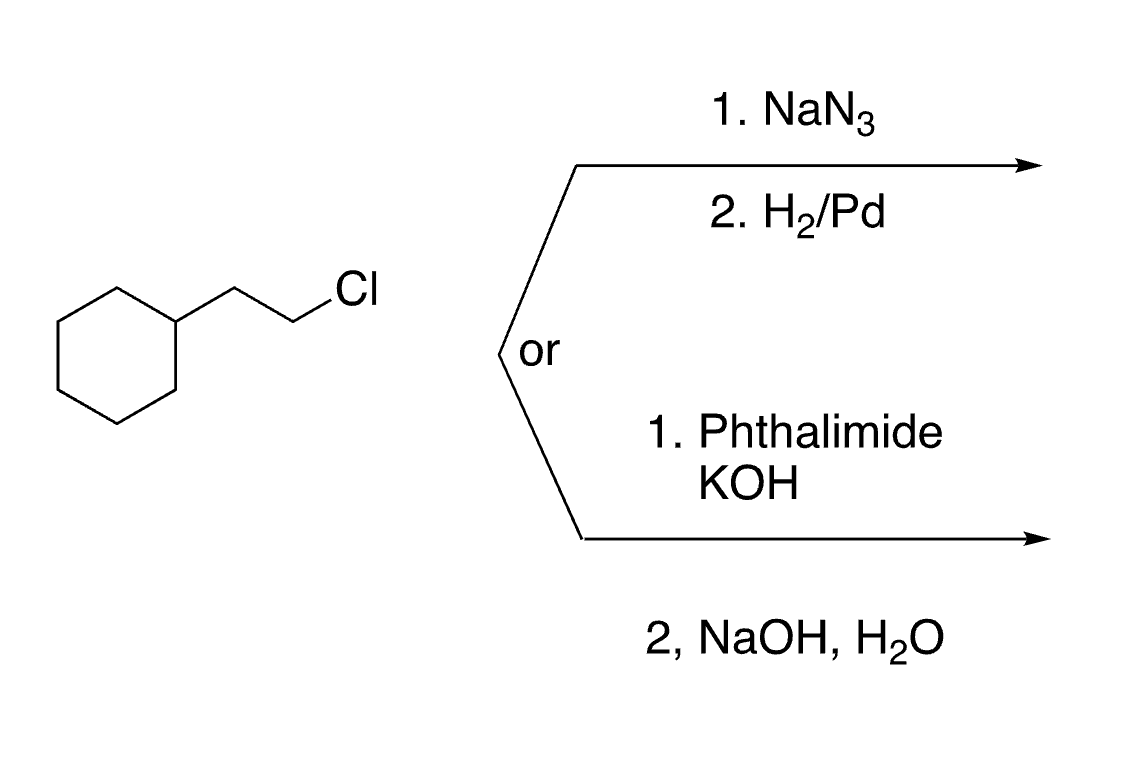
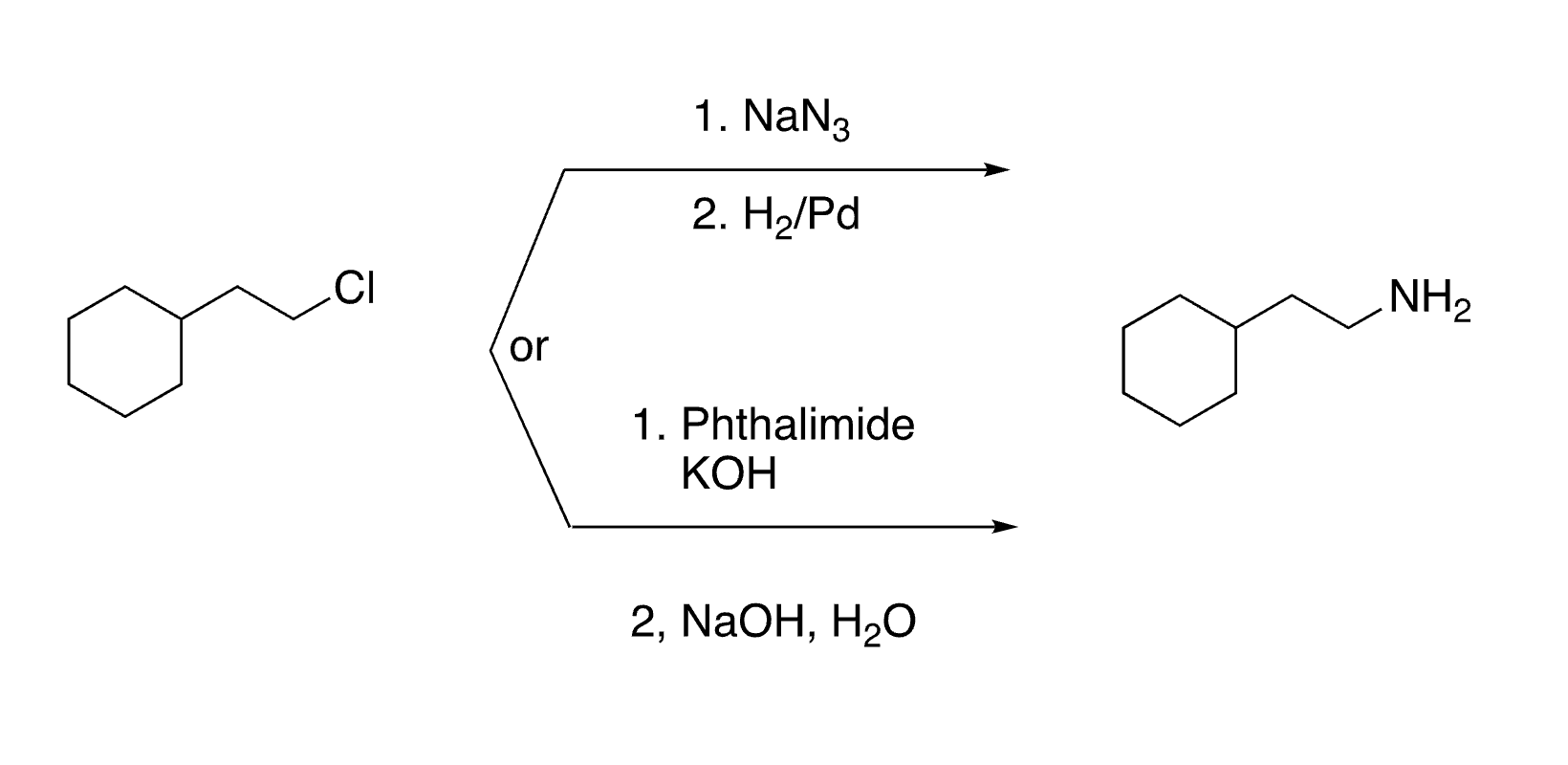
Organic Chemistry II Final Review Content (Aromatic, Aldehydes, Ketones, Amines)
0.0(0)
Card Sorting
1/96
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
1
New cards
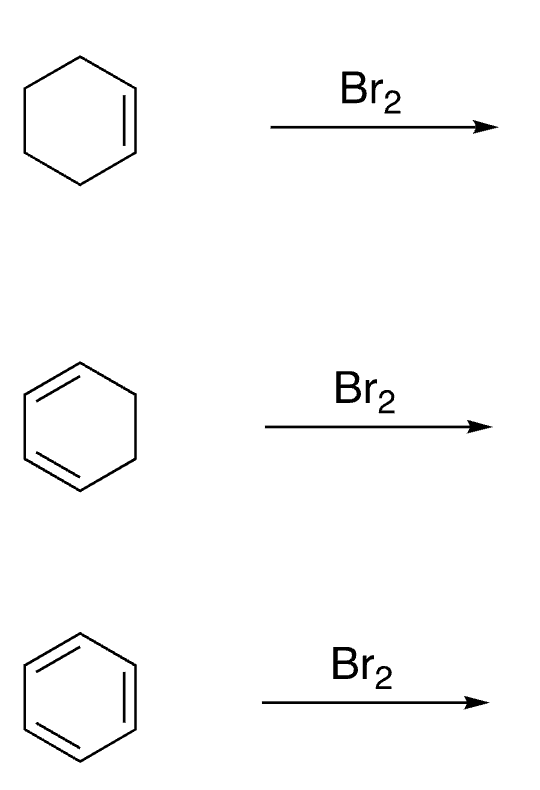
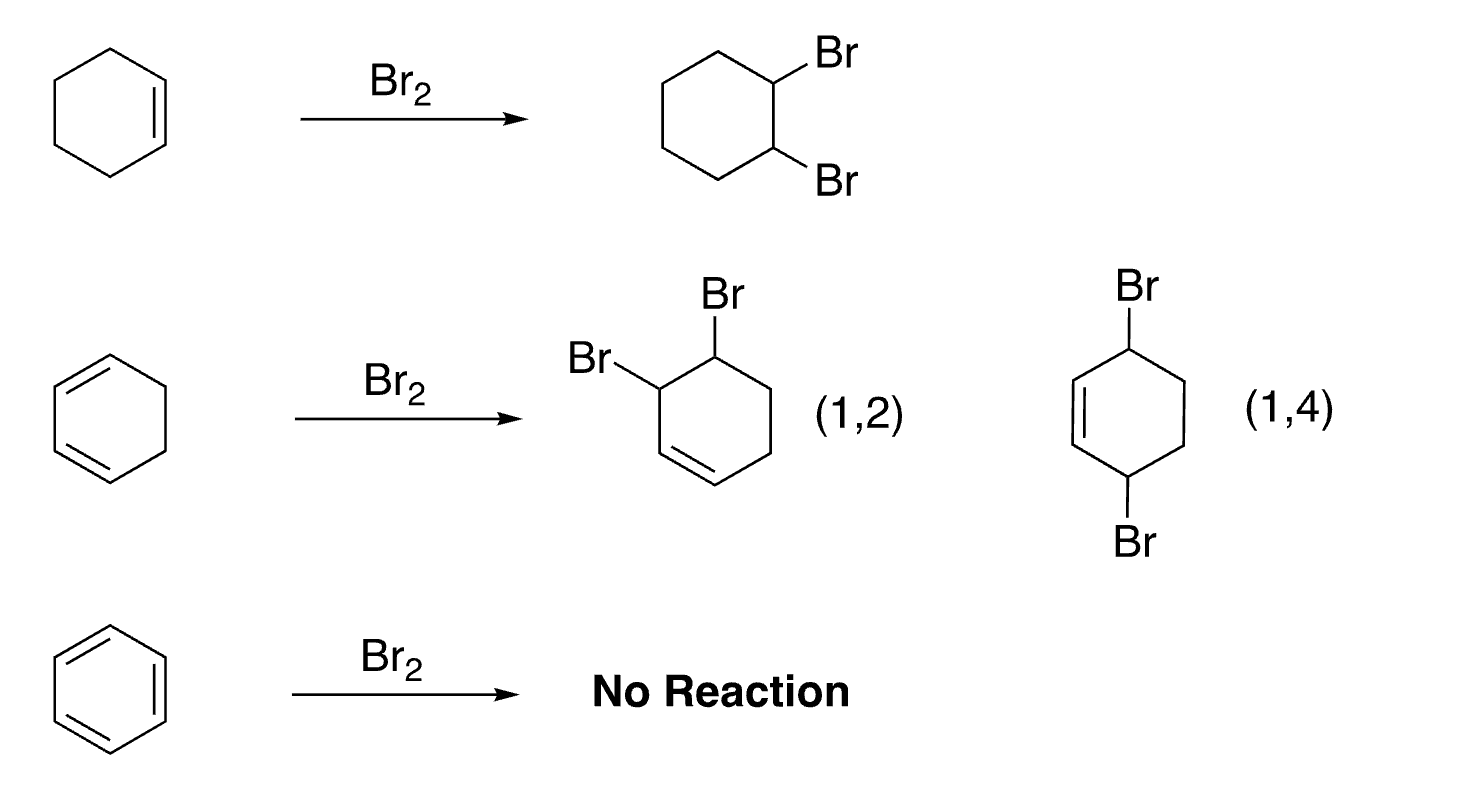
2
New cards
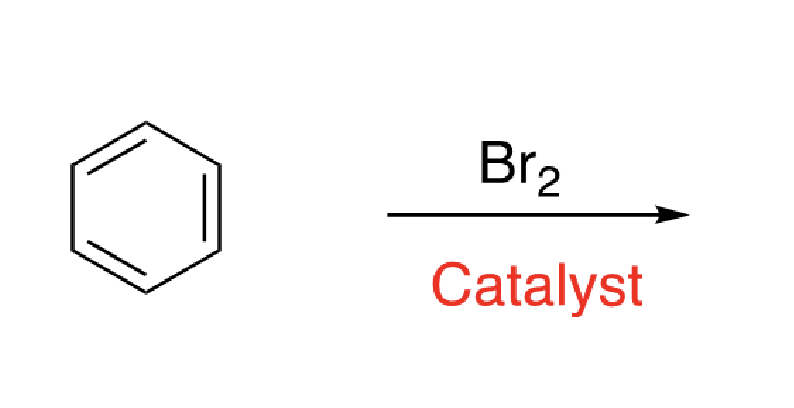
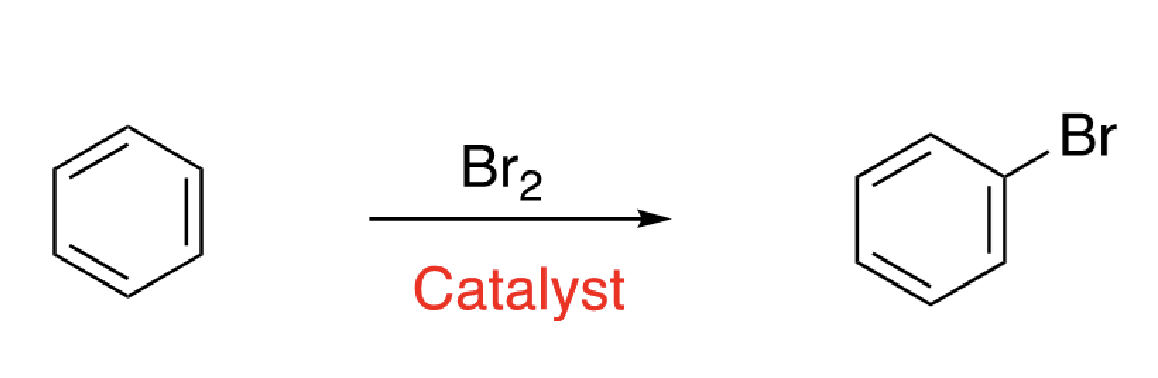
3
New cards
aromatic compound rules
1) cyclic and planar
2) all atoms must be conjugated
3) 4n+2 pi electrons (4n is anti-aromatic)
4
New cards
how many electrons in a -,+ or lone electron for aromatic compounds
-: 2
+: 0
lone electron: 1
all of these conjugate their position
5
New cards
disubstituted benzenes
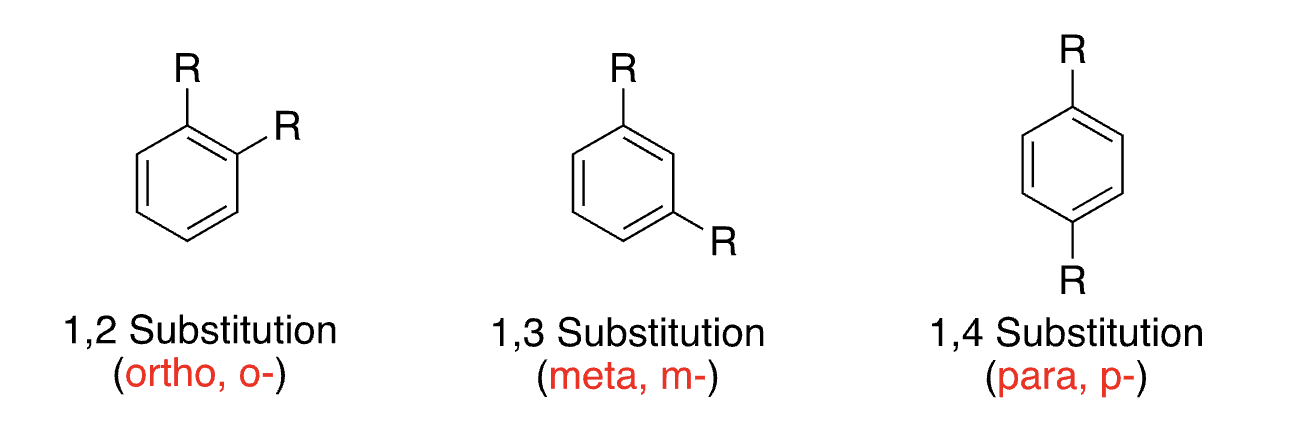
6
New cards
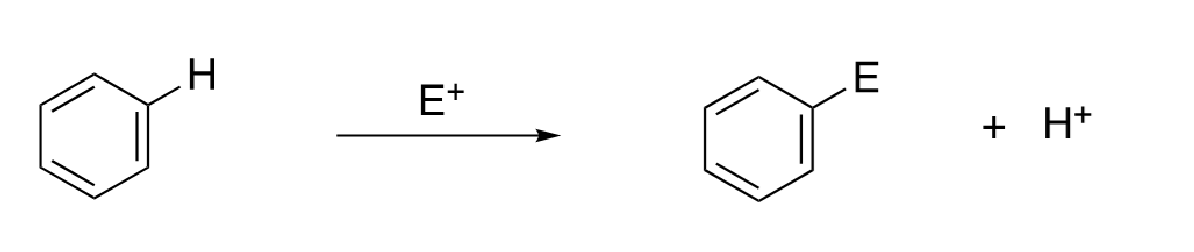
7
New cards
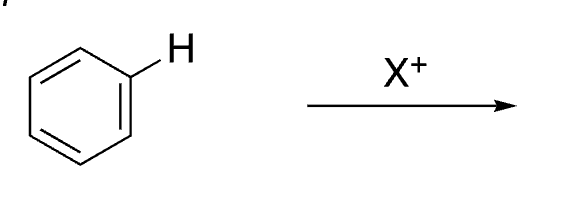
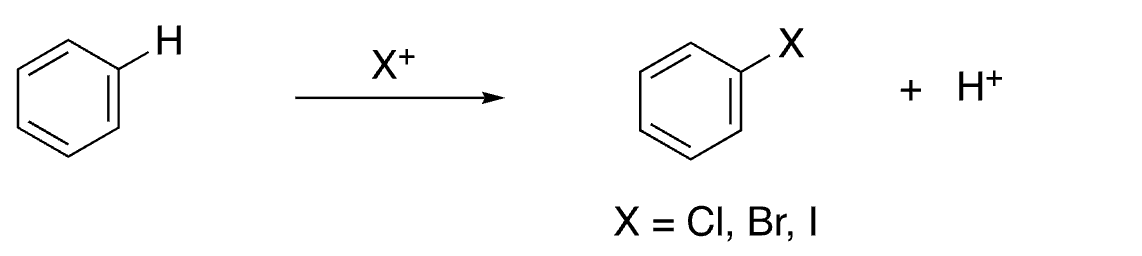
8
New cards
9
New cards
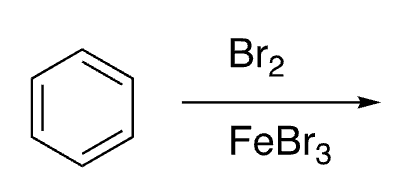
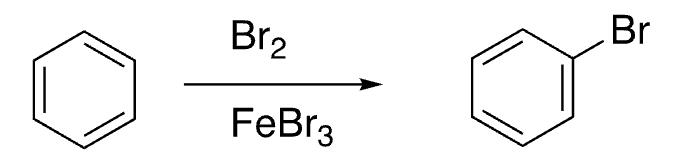
10
New cards
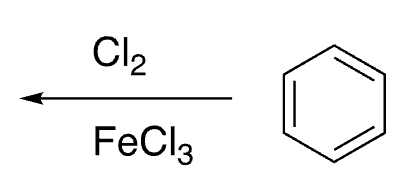
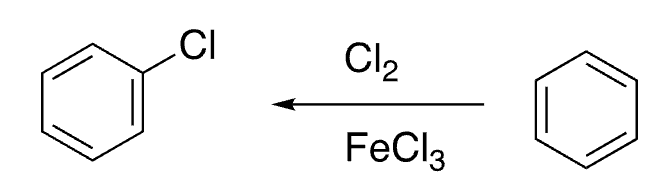
11
New cards
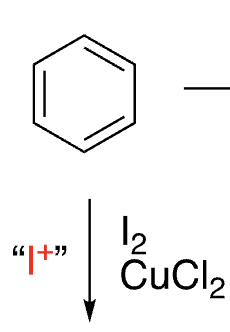
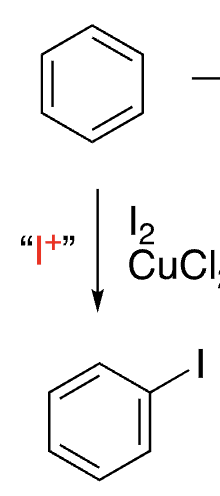
12
New cards
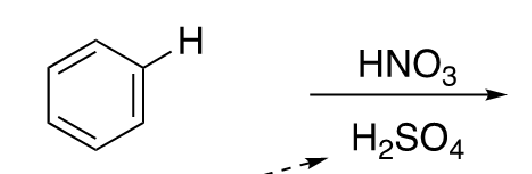
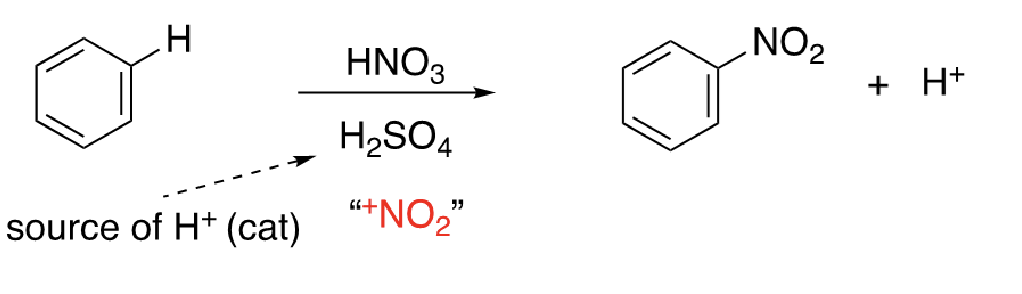
13
New cards
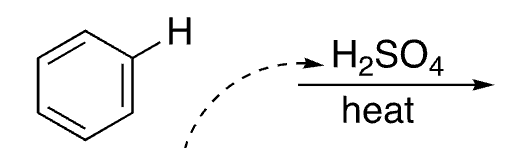
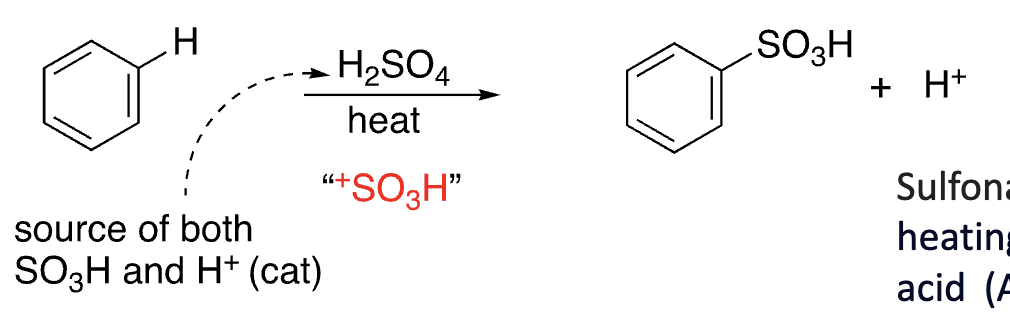
14
New cards
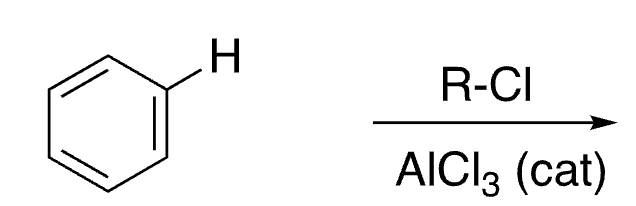
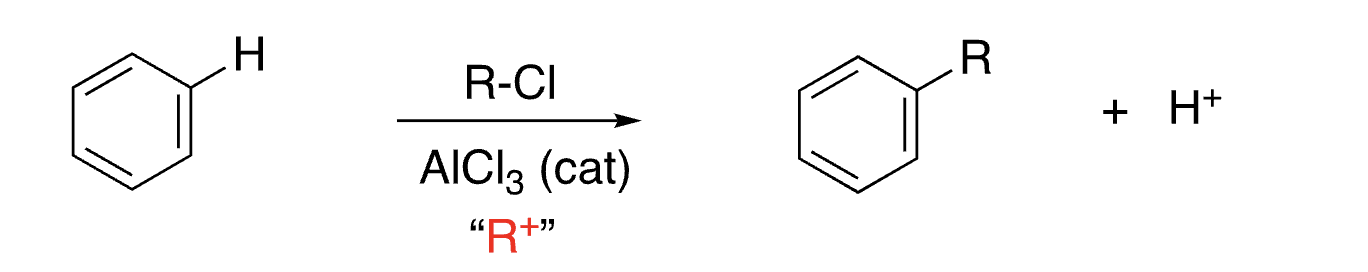
15
New cards
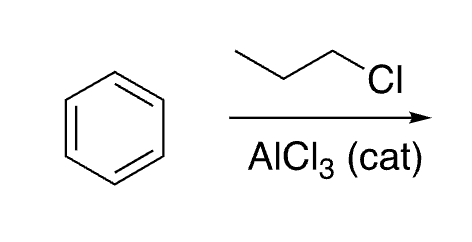
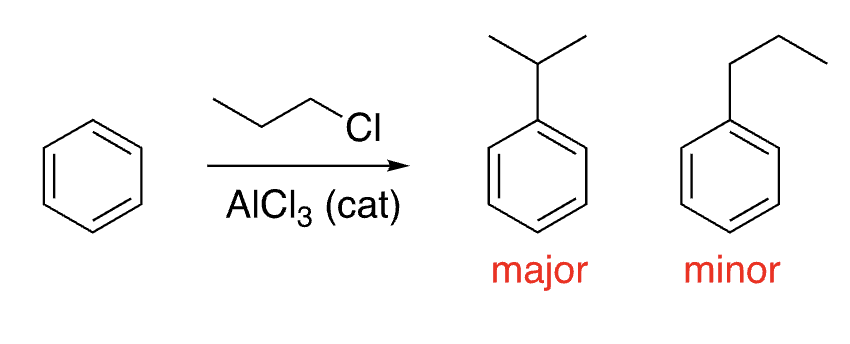
16
New cards
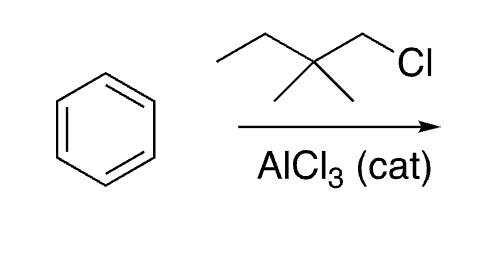
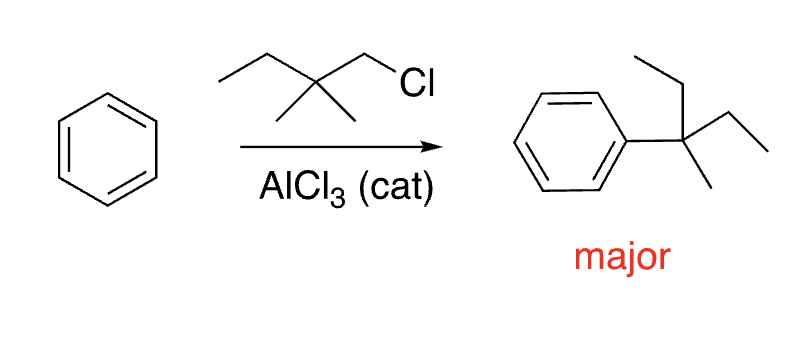
17
New cards
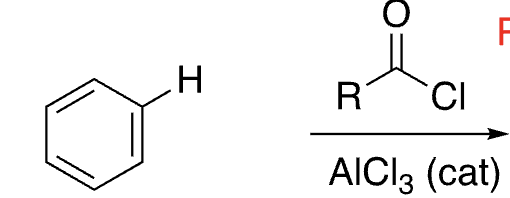
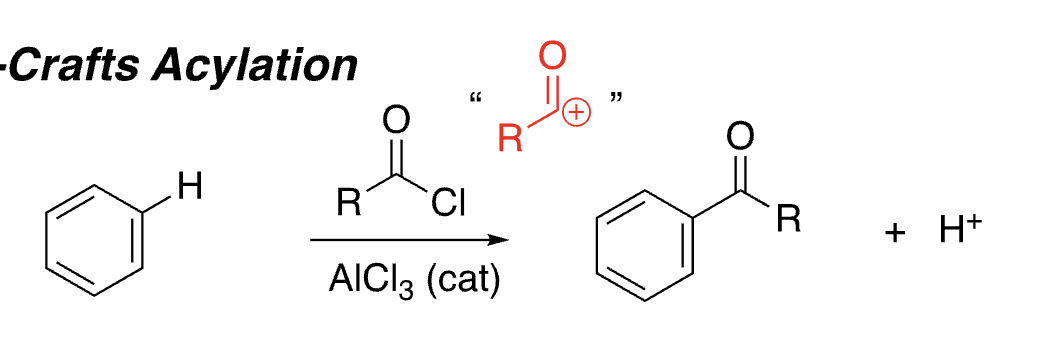
18
New cards
do electron donating or withdrawing groups produce faster reactions
electron donating (strongest nucleophile)
19
New cards
electron donating groups are _____ directors
ortho and para
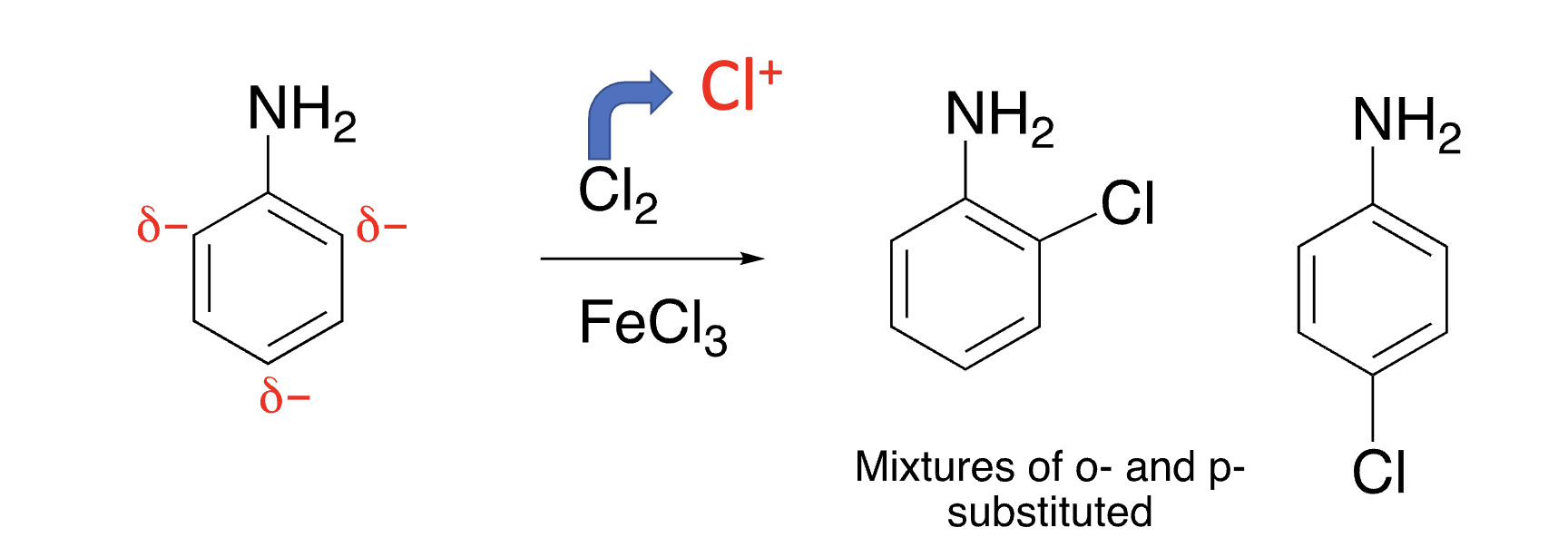
20
New cards
electron withdrawing groups are ____ directors
meta
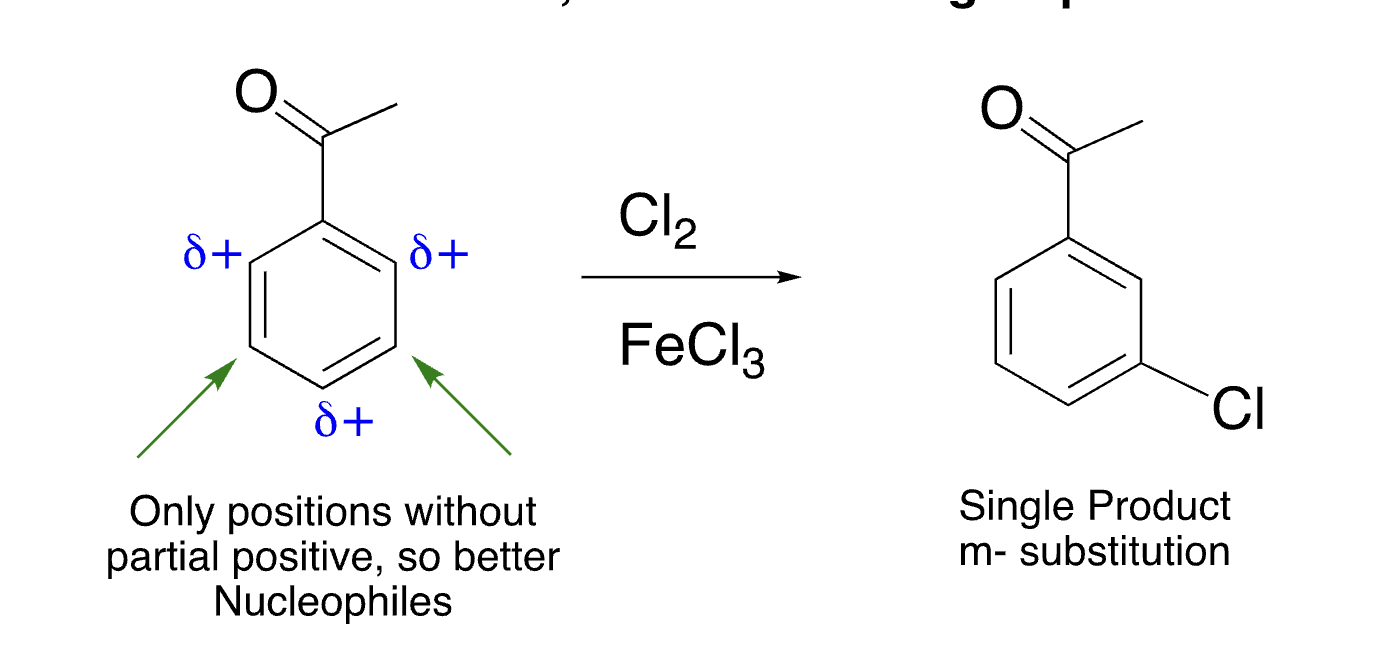
21
New cards
list of o/p directors
OH, SH, NH2, OR, NHR, RO2, halogens
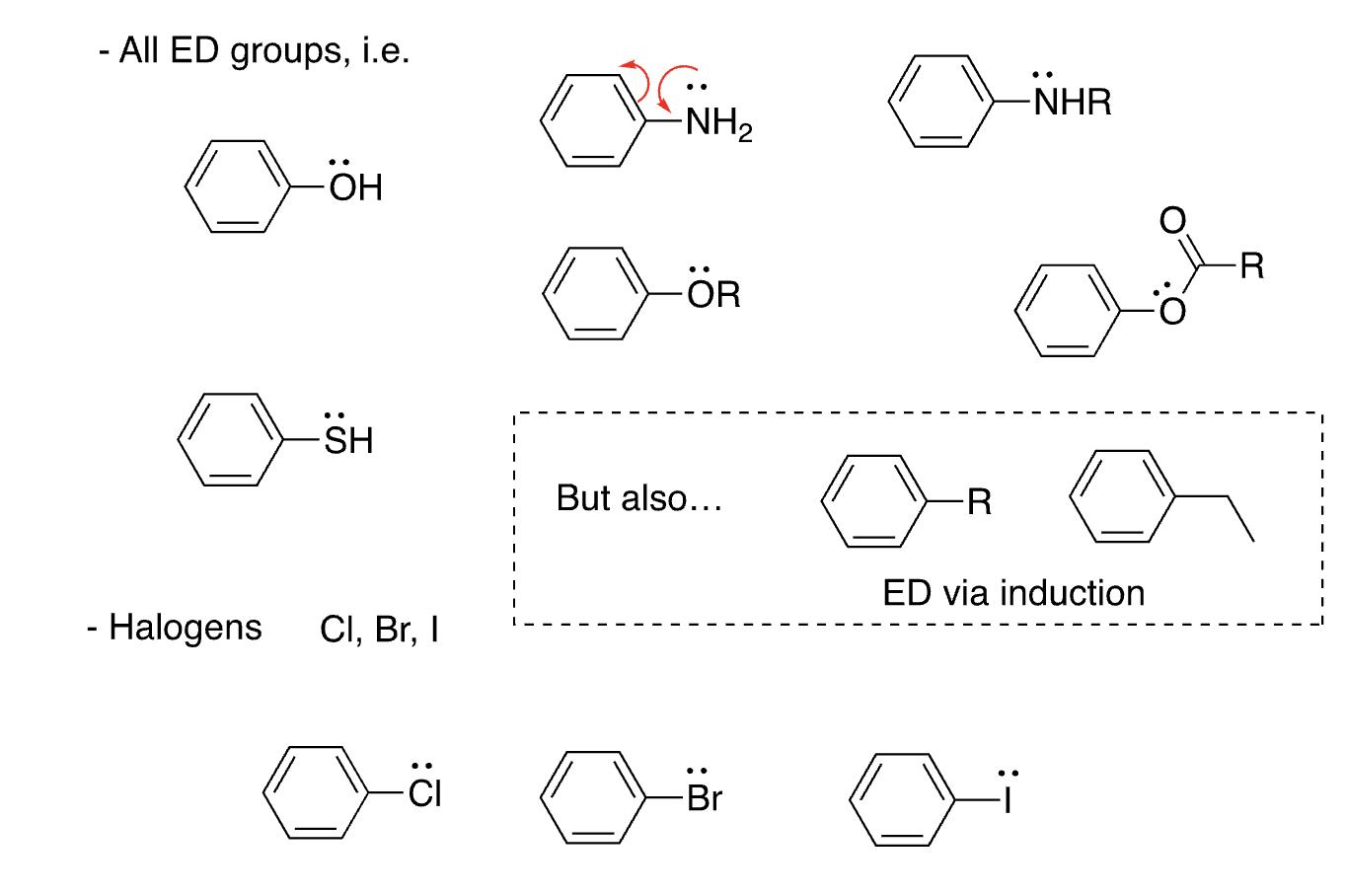
22
New cards
list of meta directors
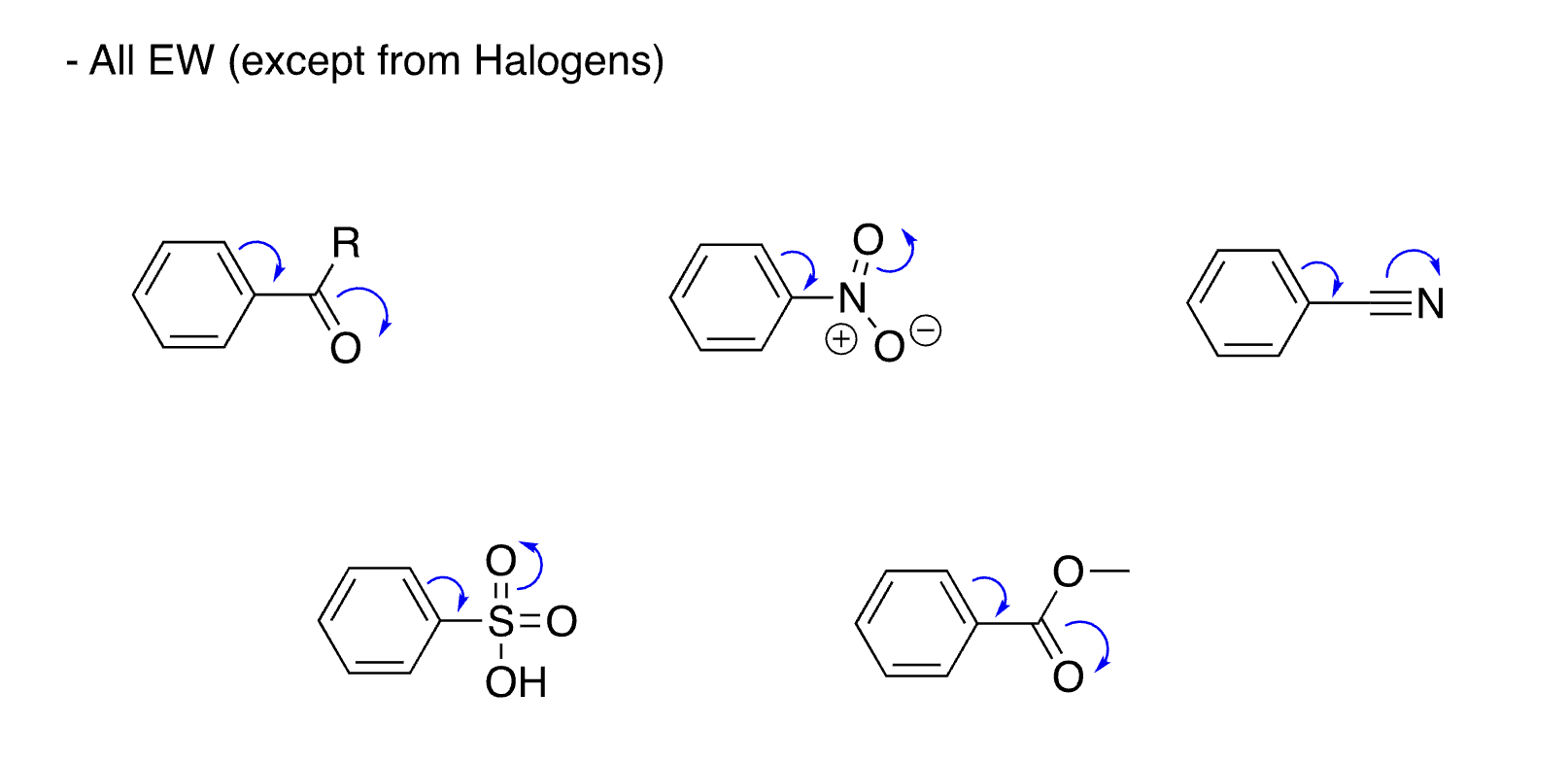
23
New cards
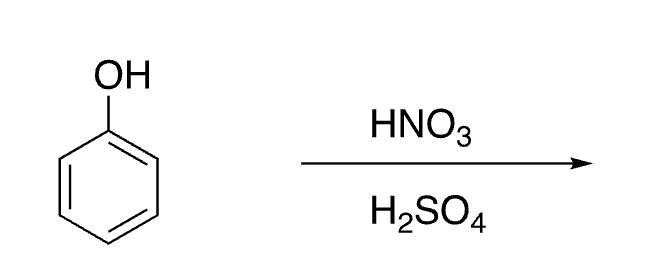
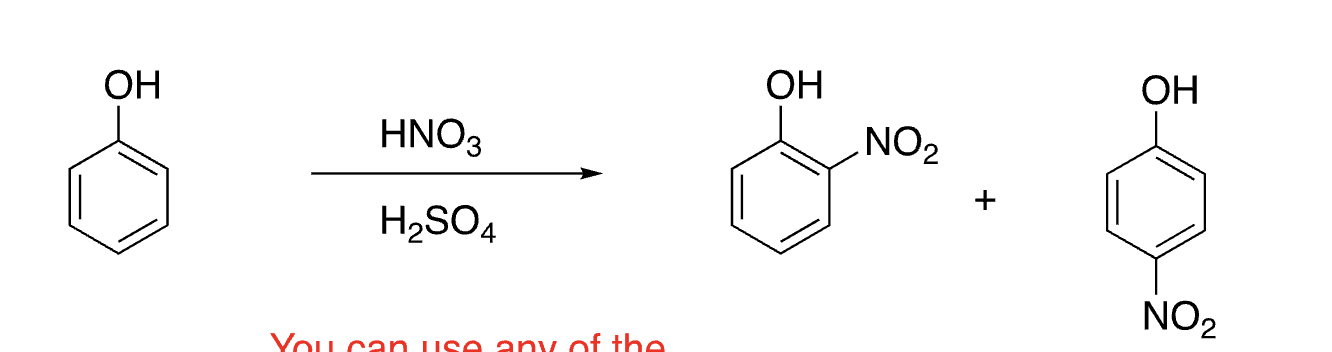
24
New cards
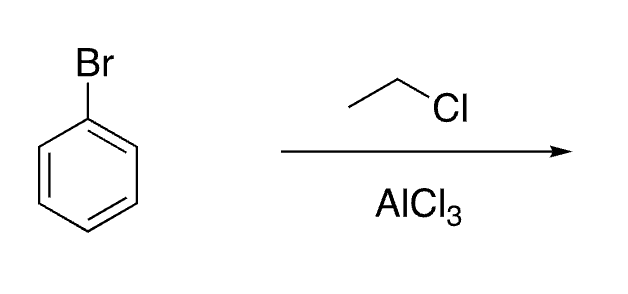
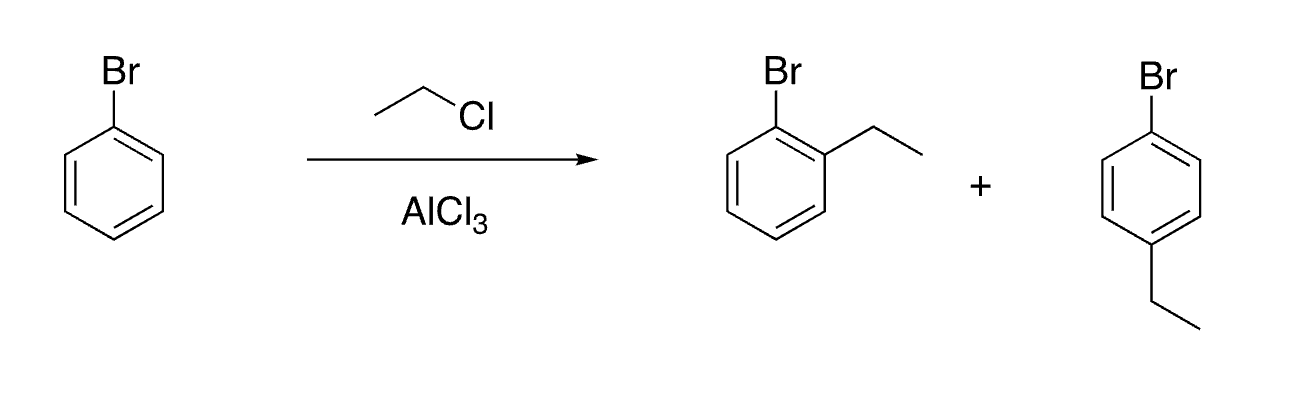
25
New cards
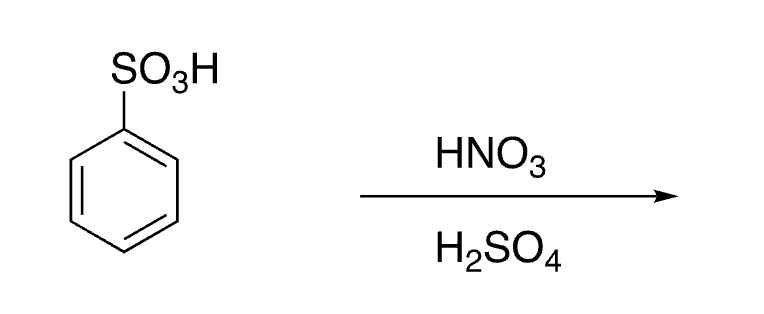
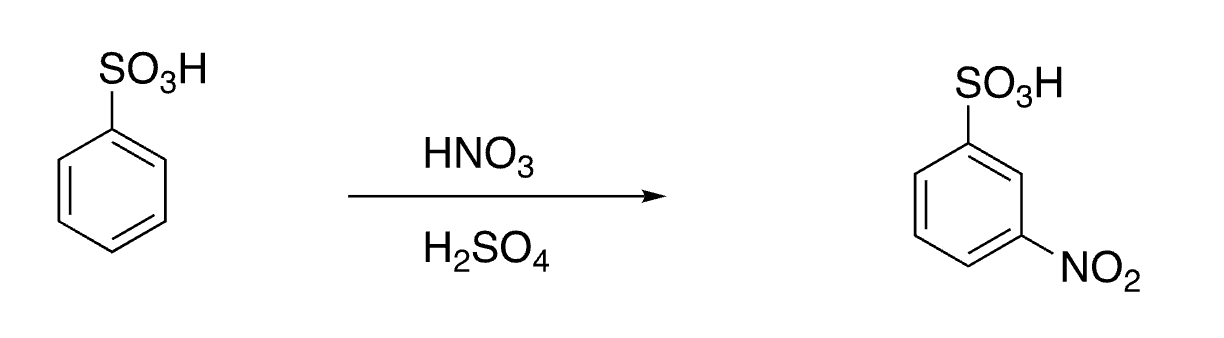
26
New cards
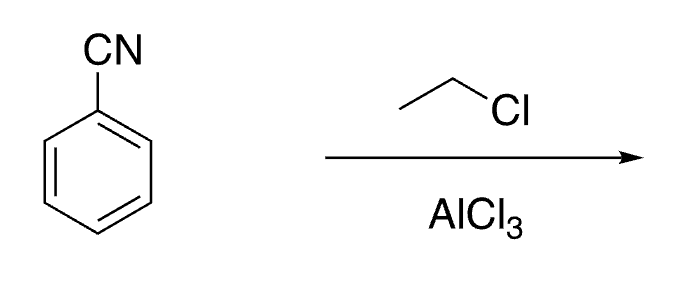
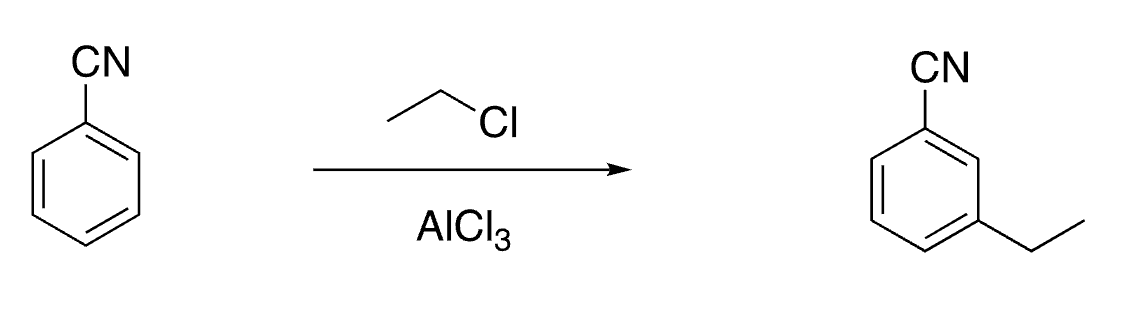
27
New cards
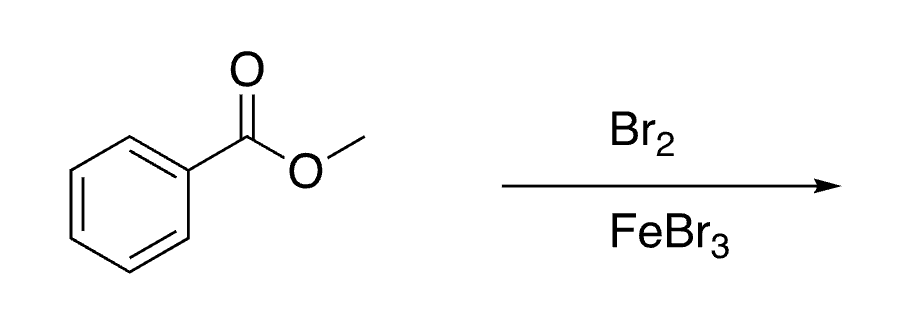
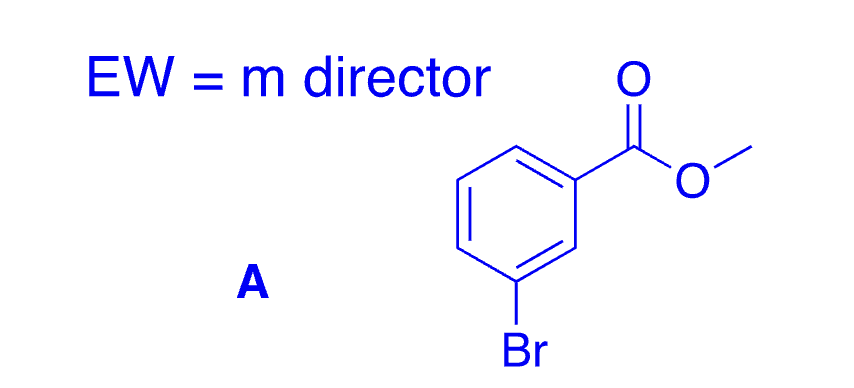
28
New cards

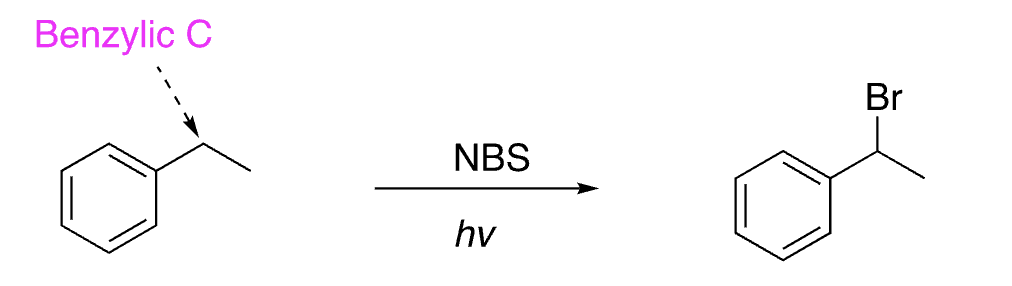
29
New cards
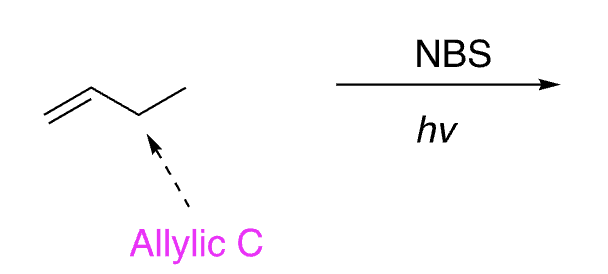
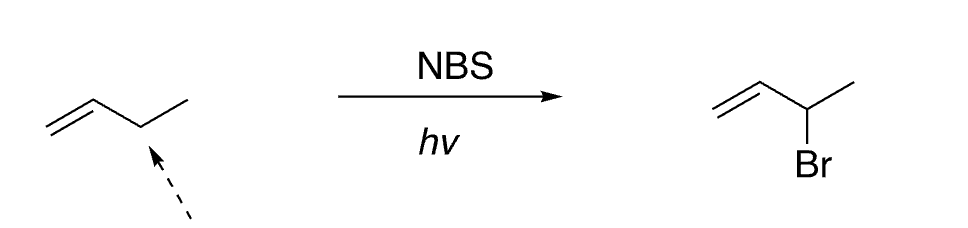
30
New cards
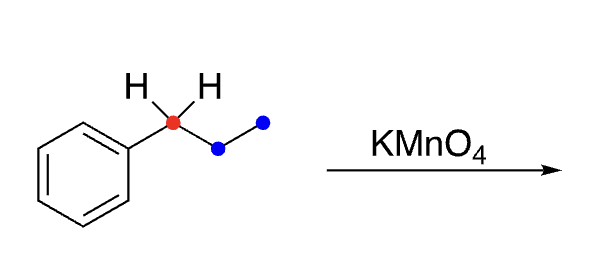
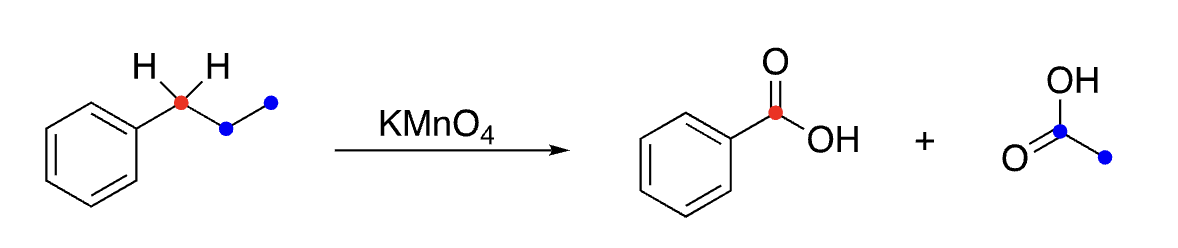
31
New cards
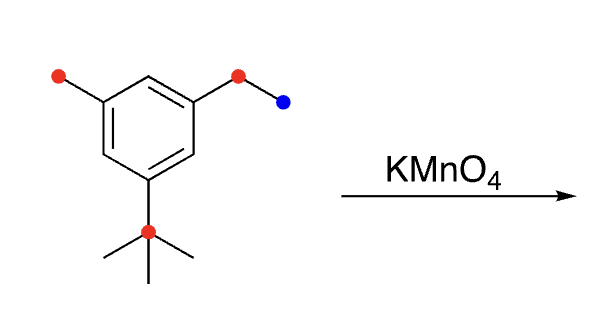
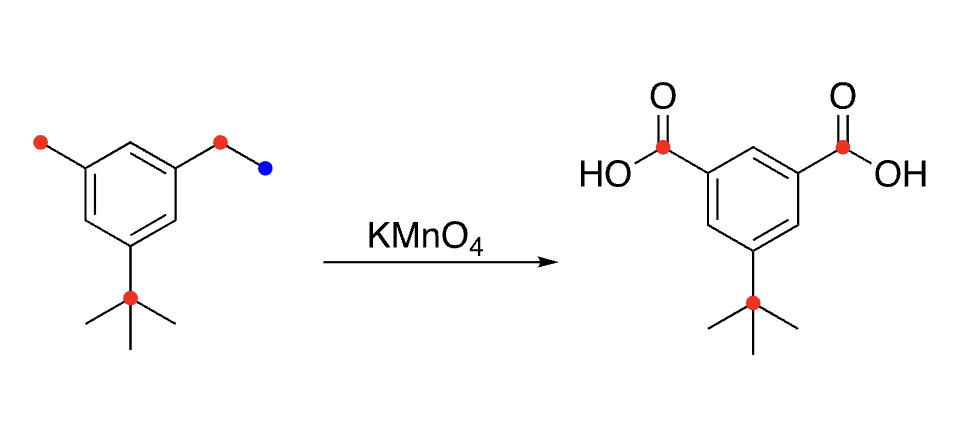
32
New cards
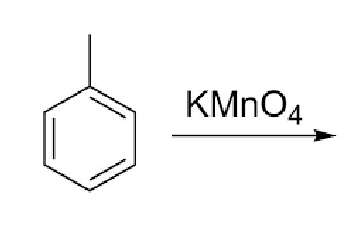
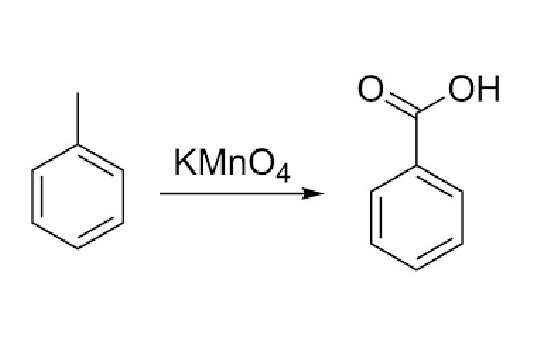
33
New cards
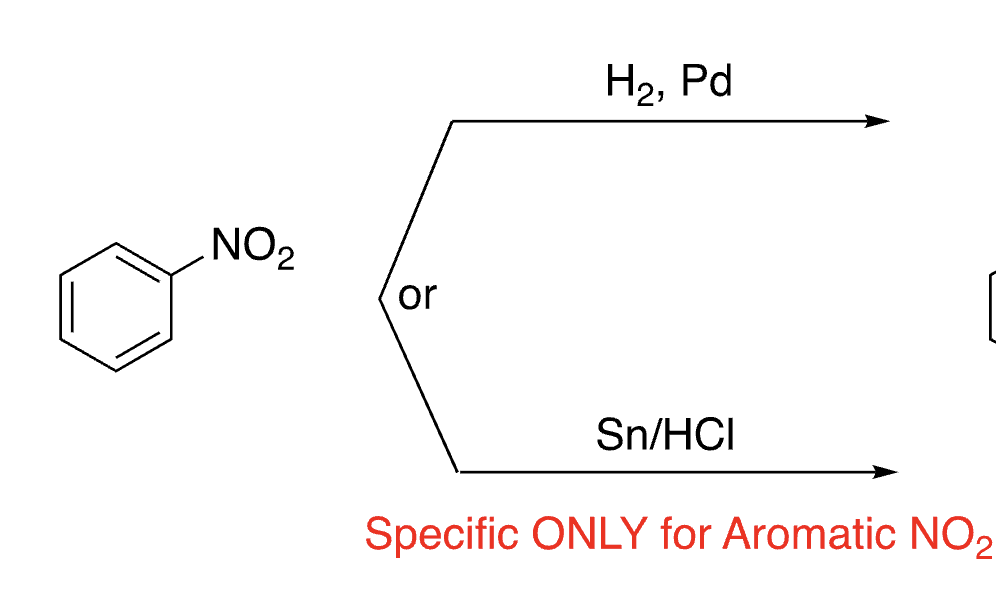
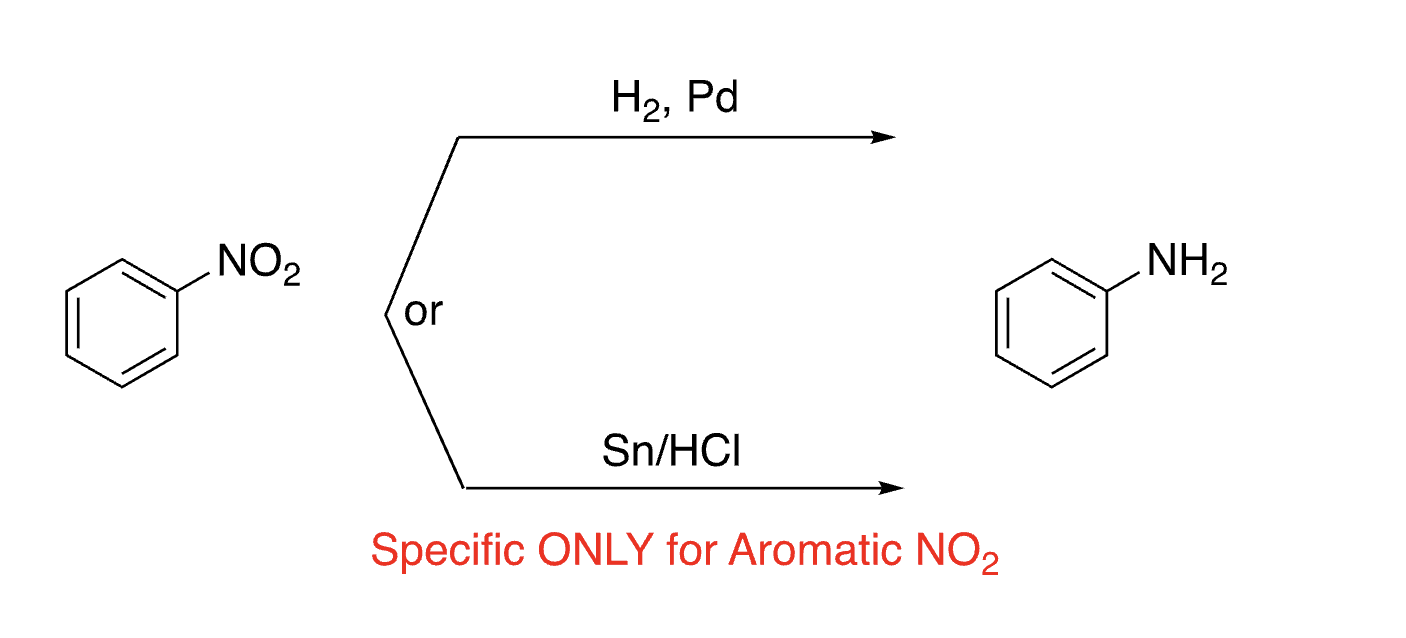
34
New cards
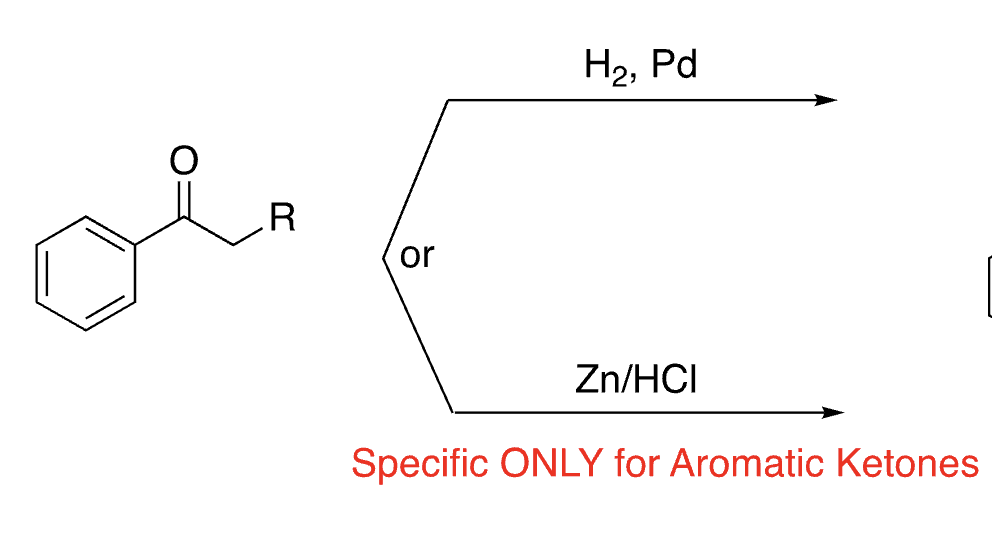
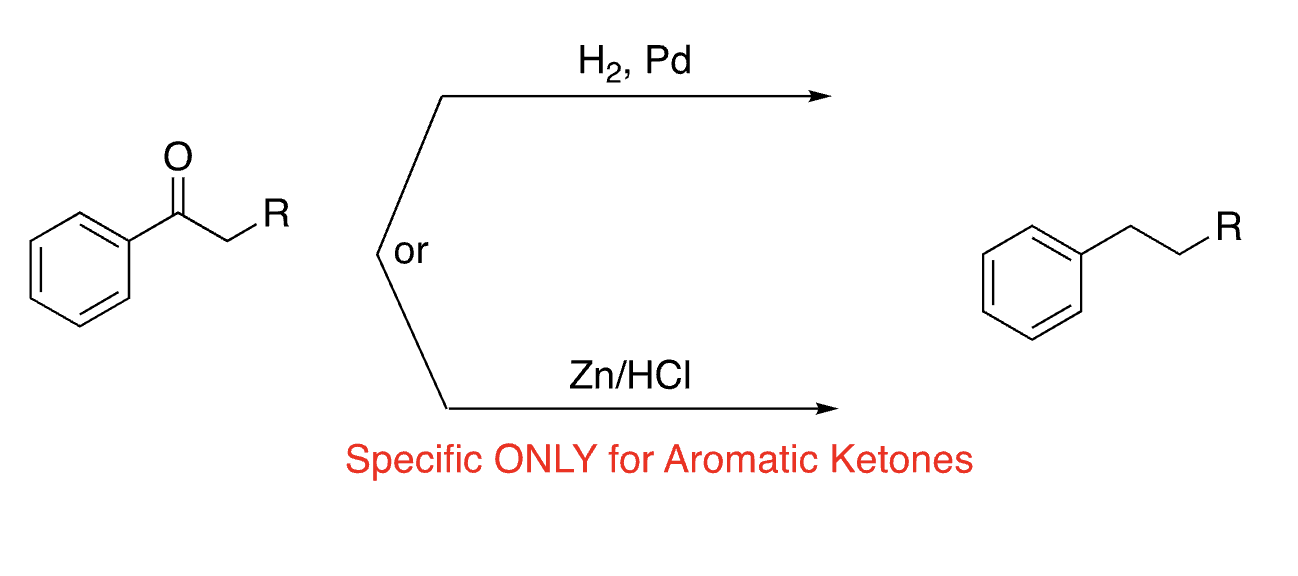
35
New cards
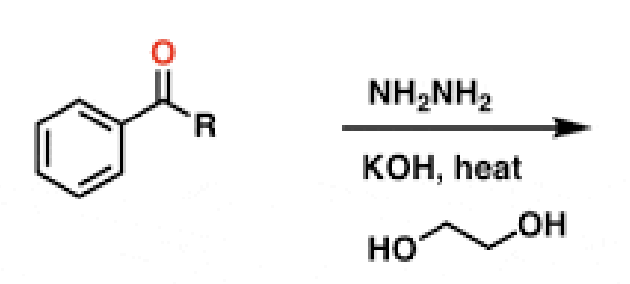
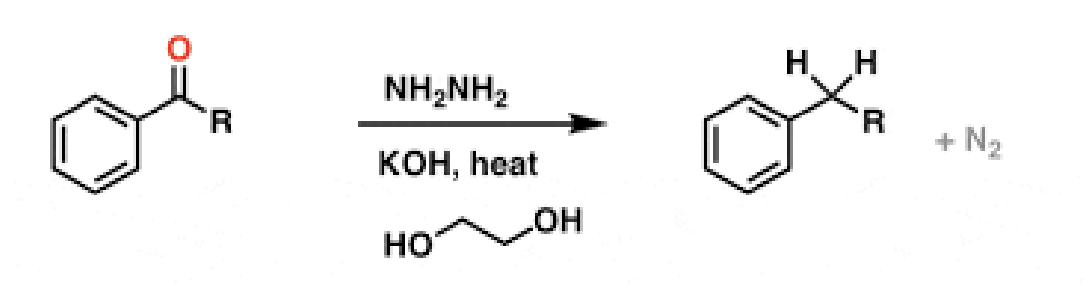
36
New cards
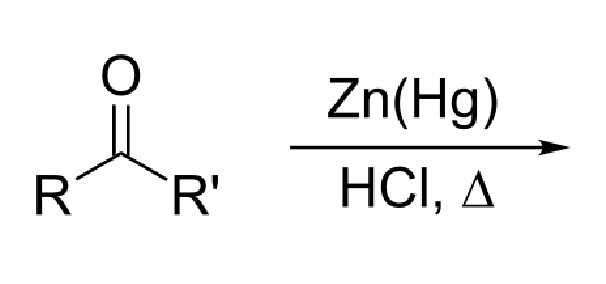
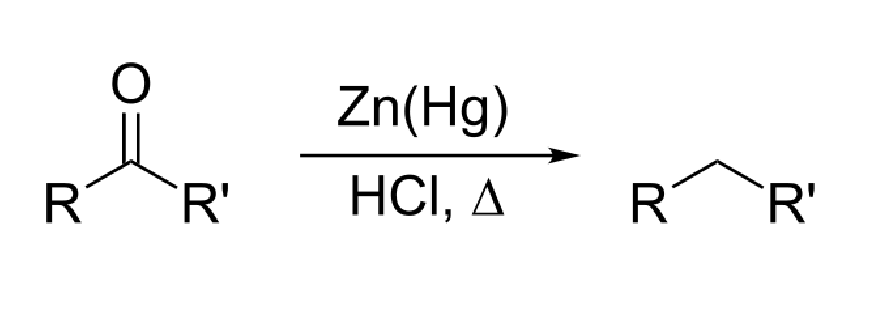
37
New cards
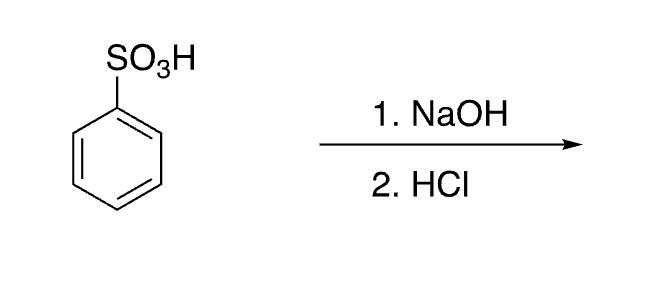
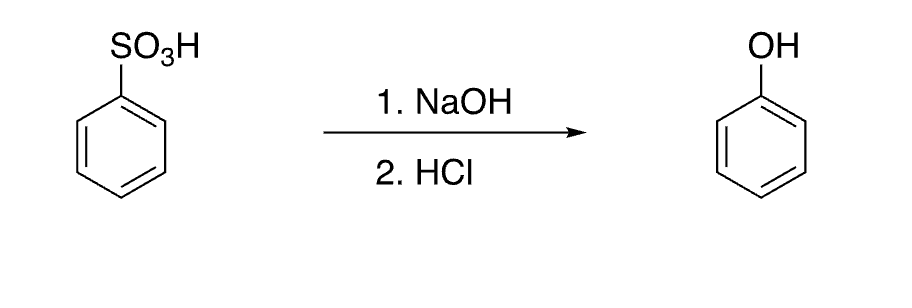
38
New cards
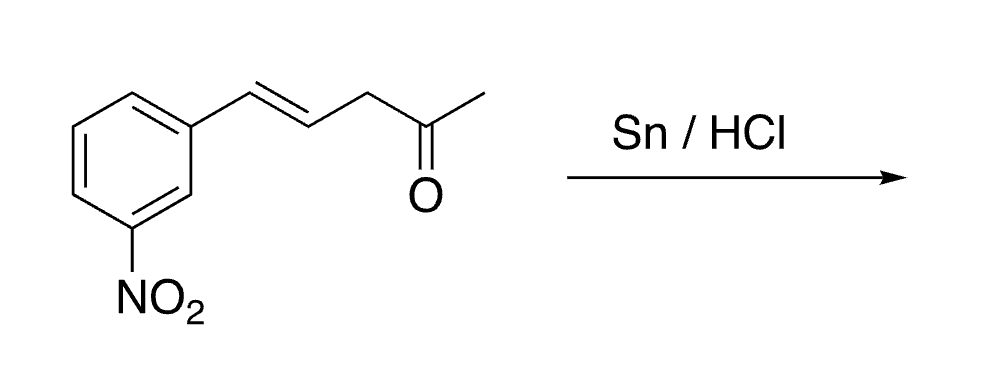
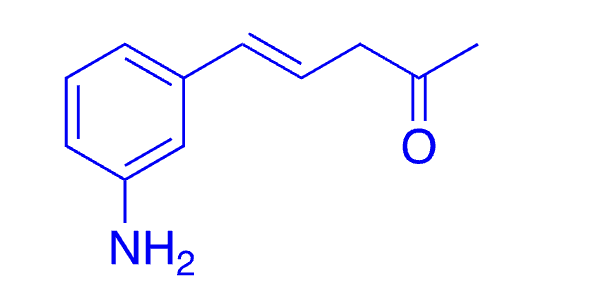
39
New cards
aldehydes vs ketones structure
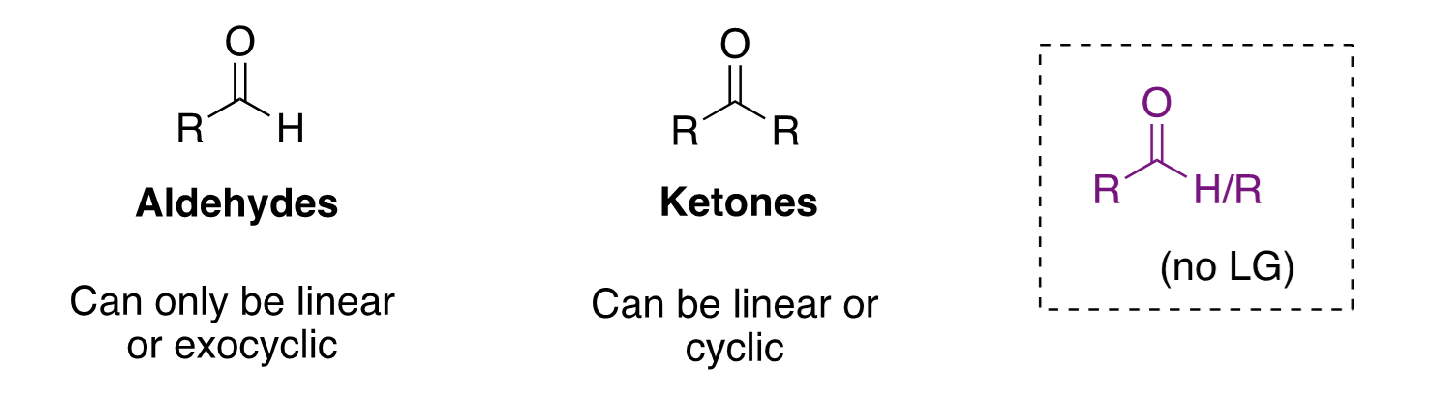
40
New cards
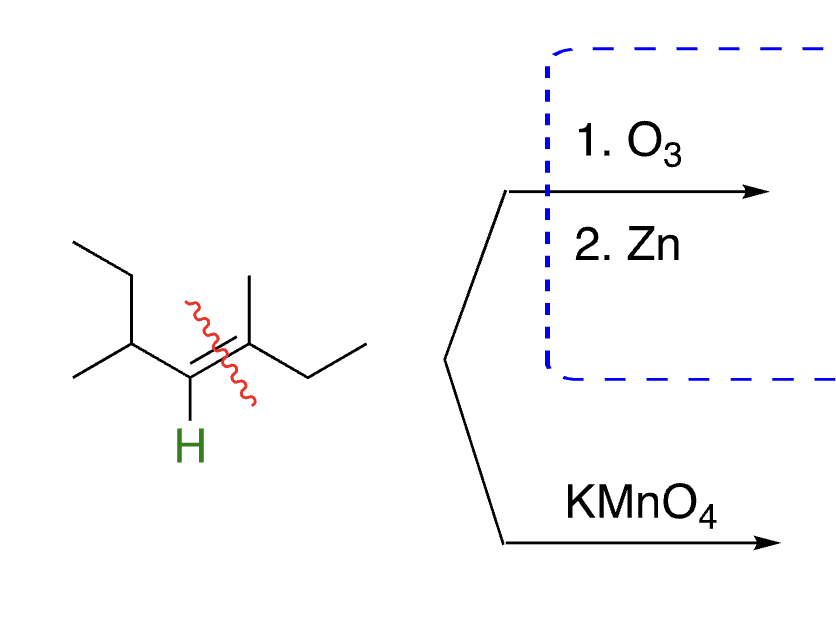
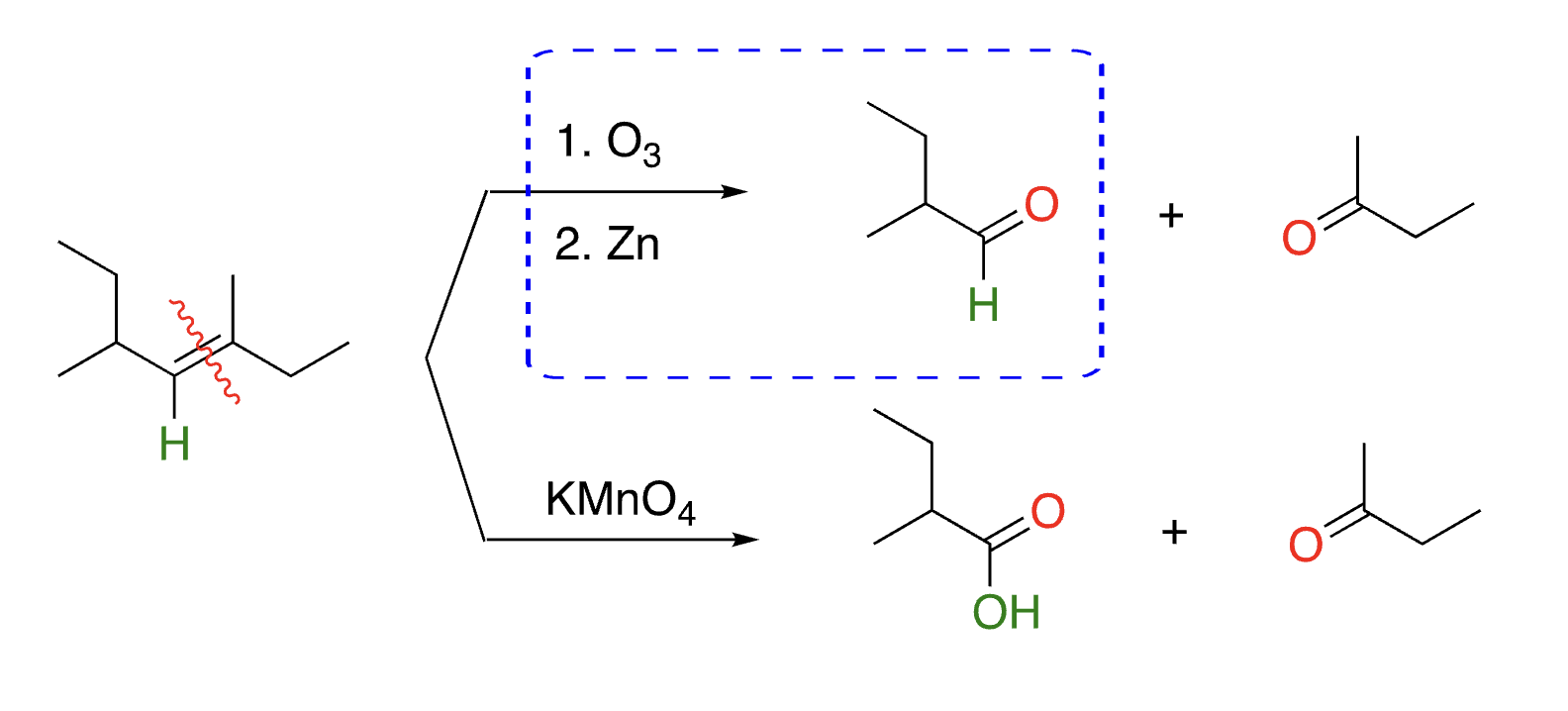
41
New cards
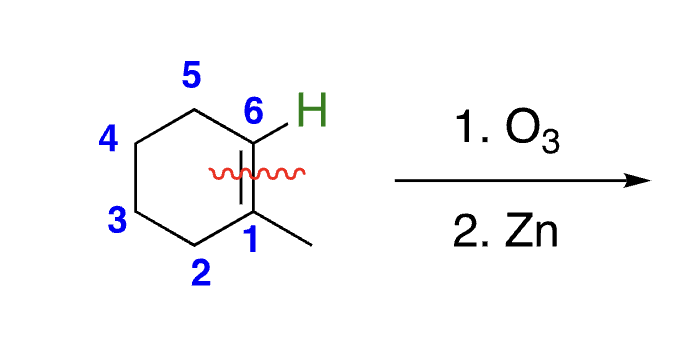
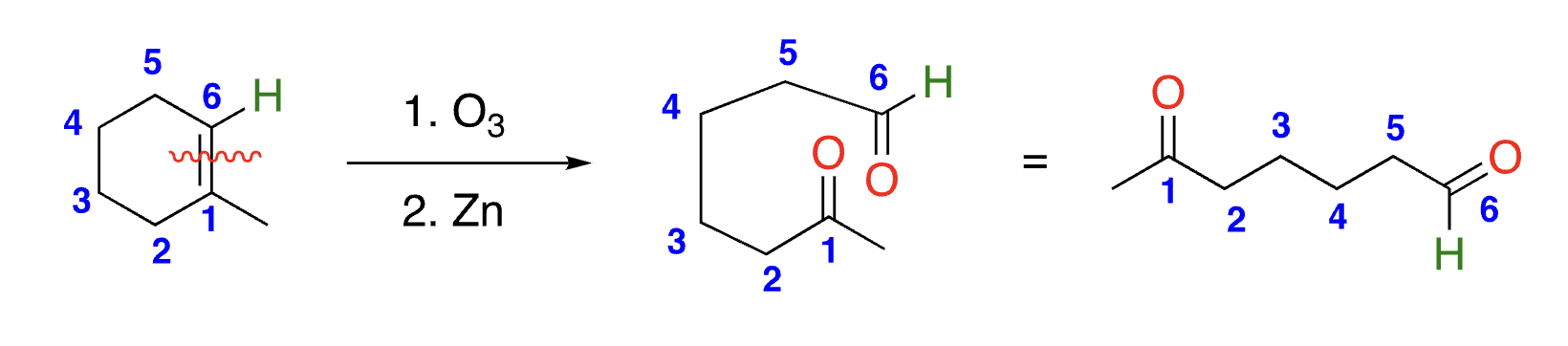
42
New cards
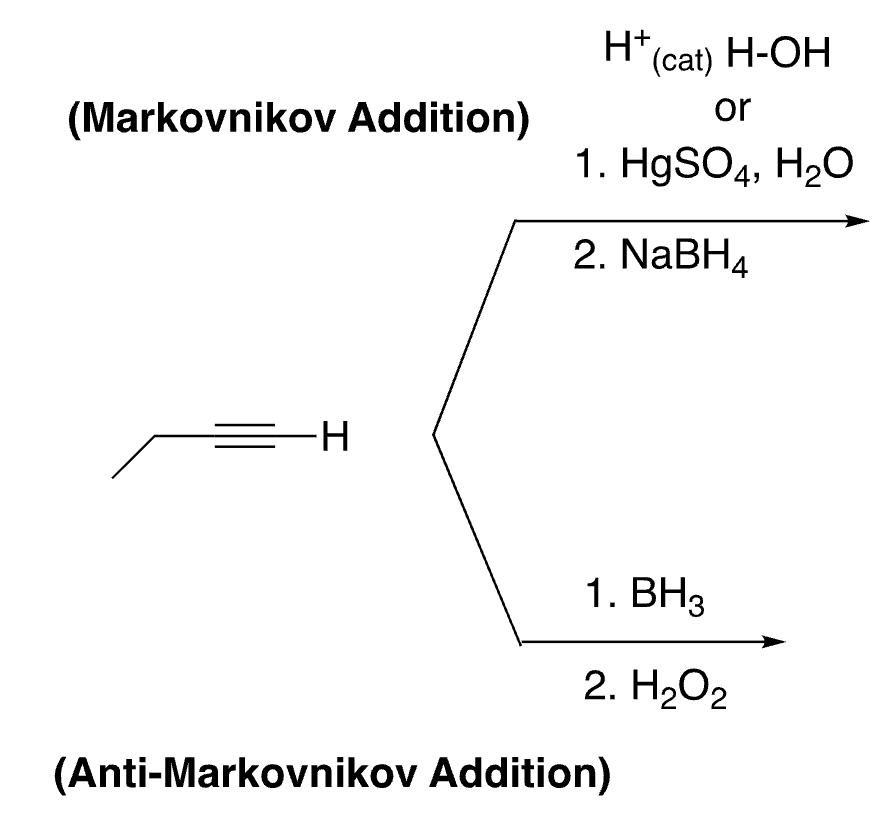
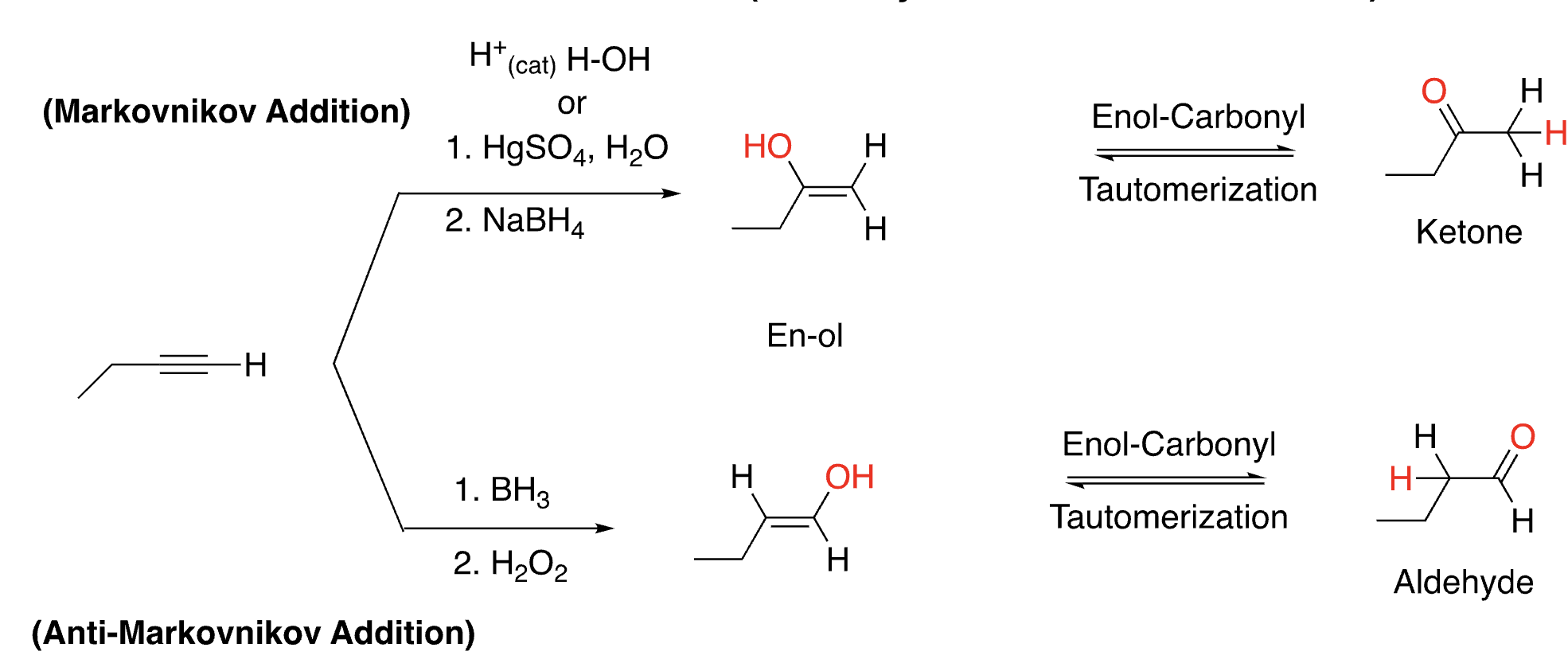
43
New cards
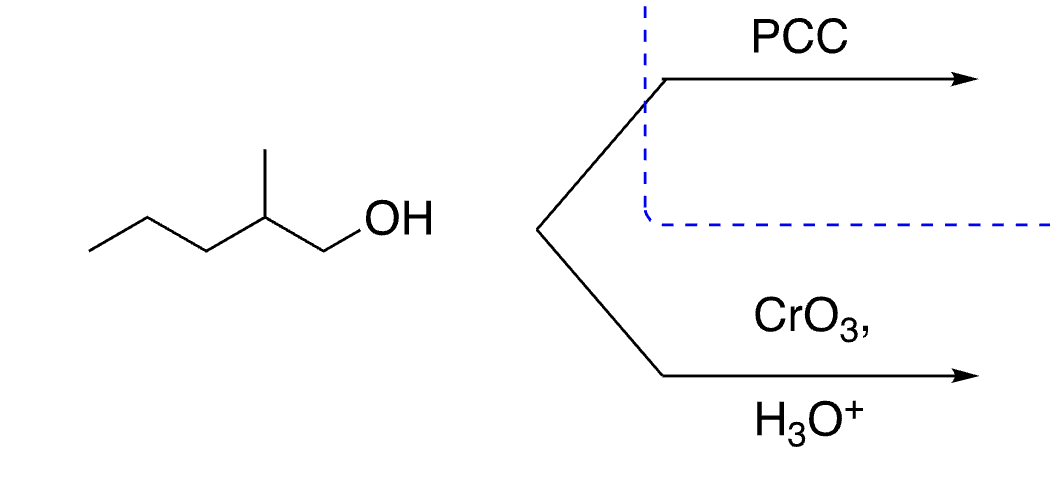
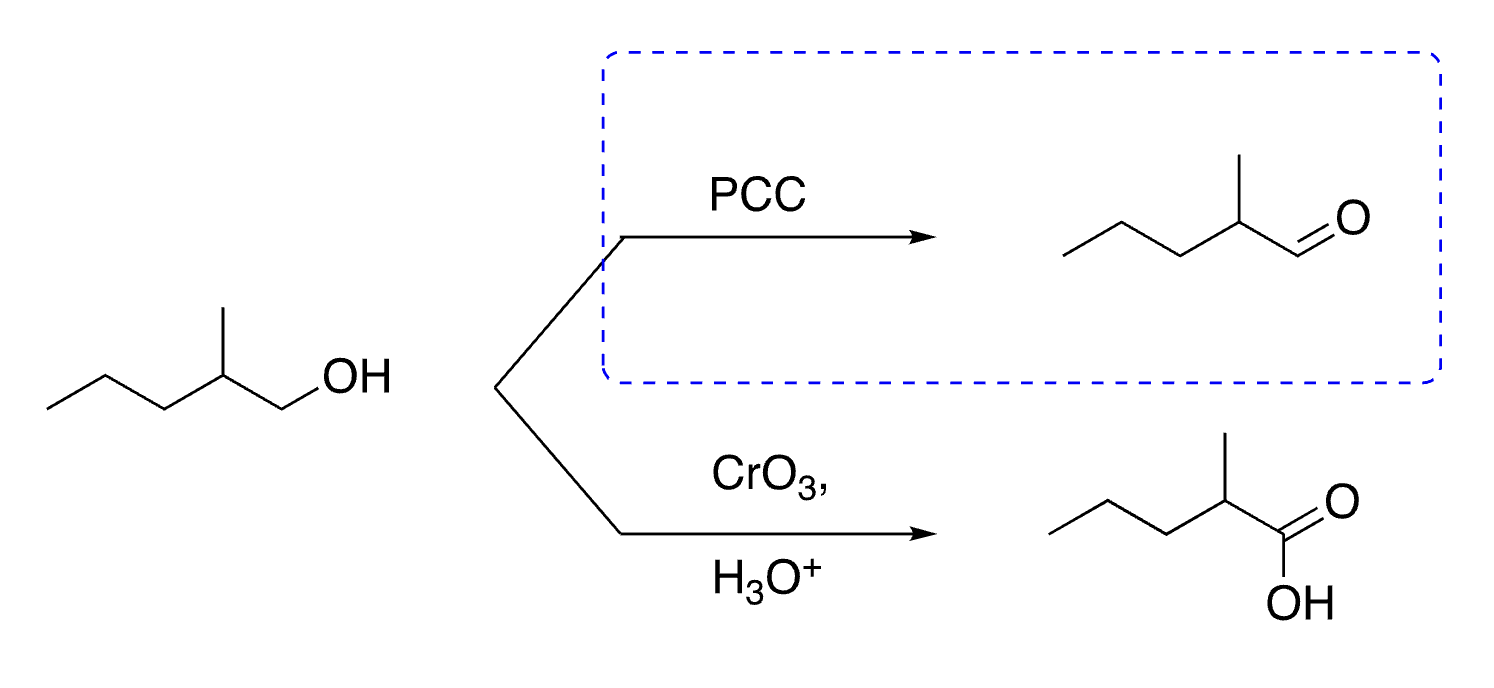
44
New cards
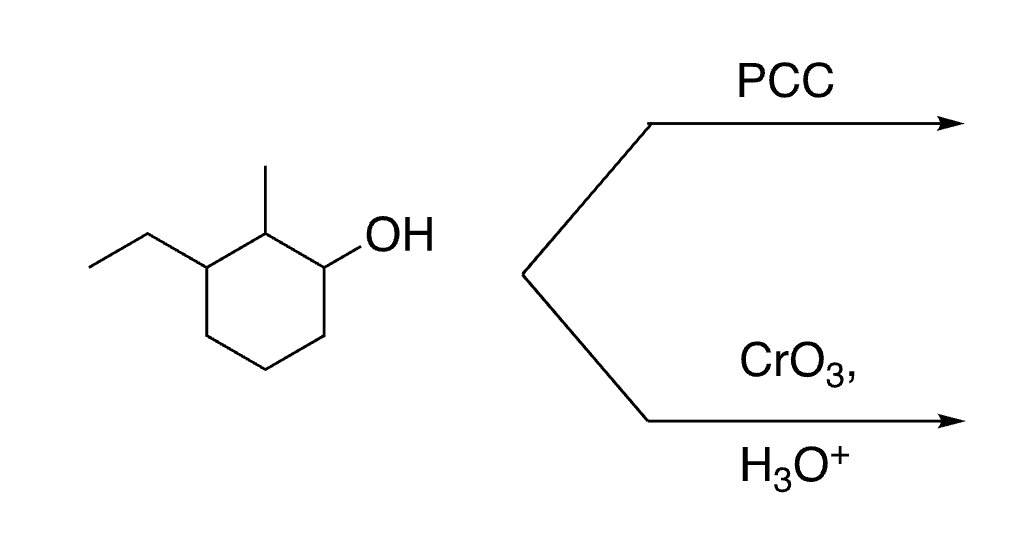
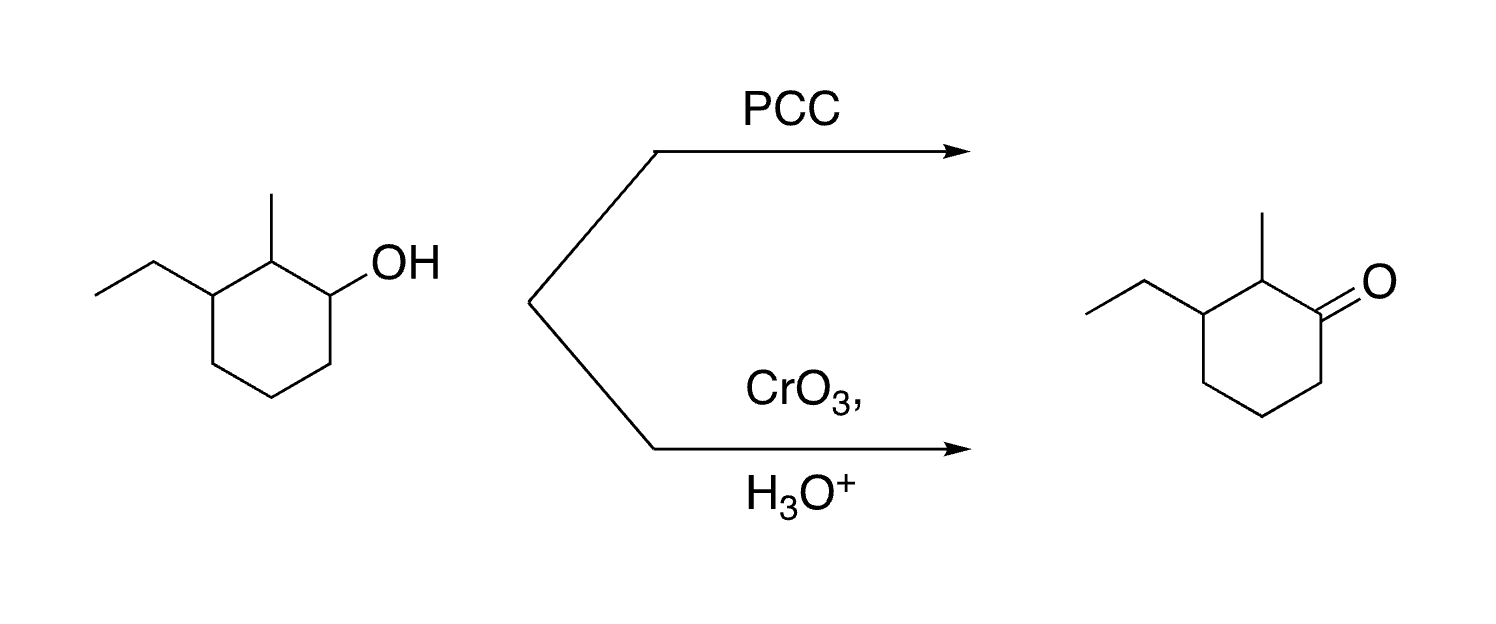
45
New cards
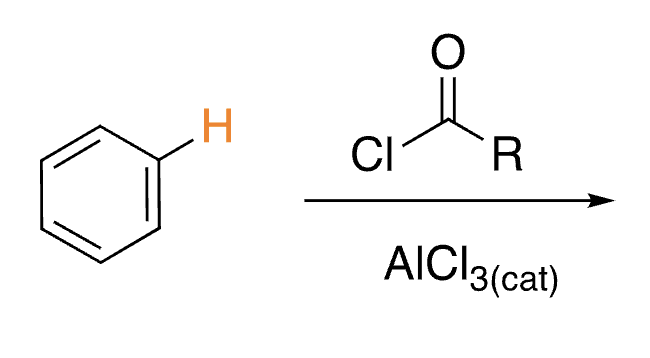
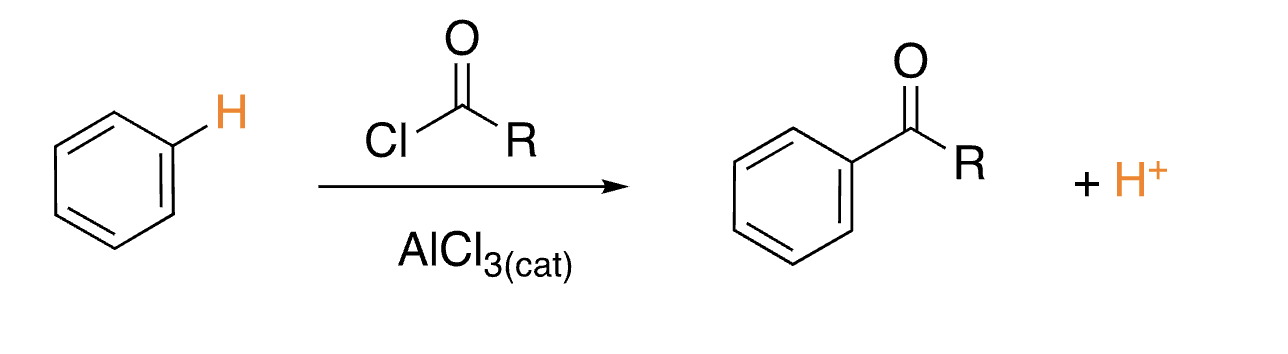
46
New cards
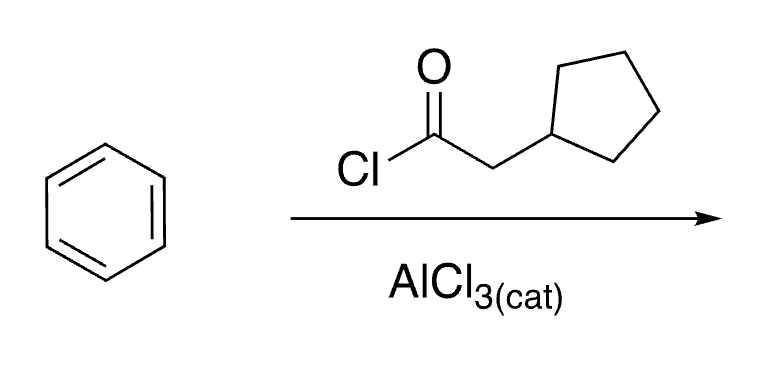
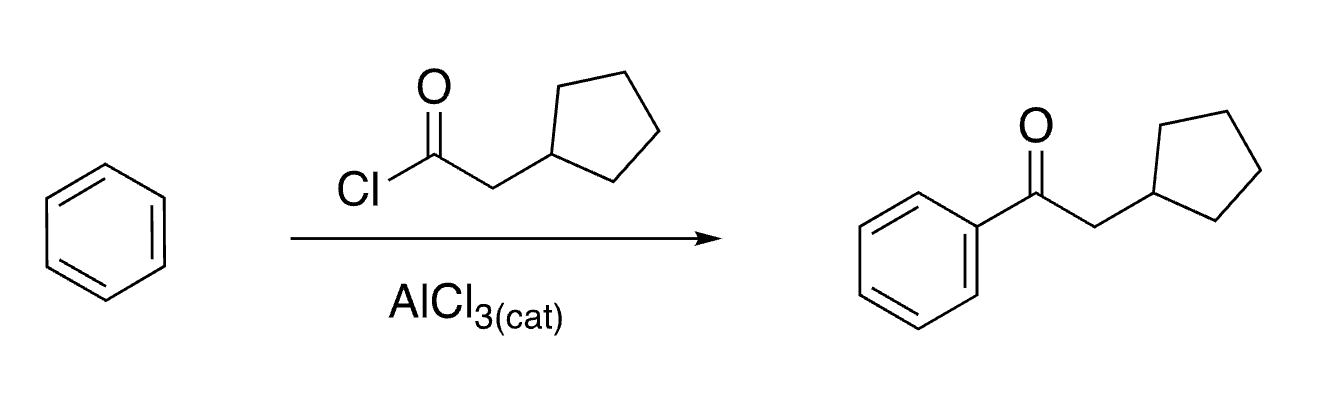
47
New cards
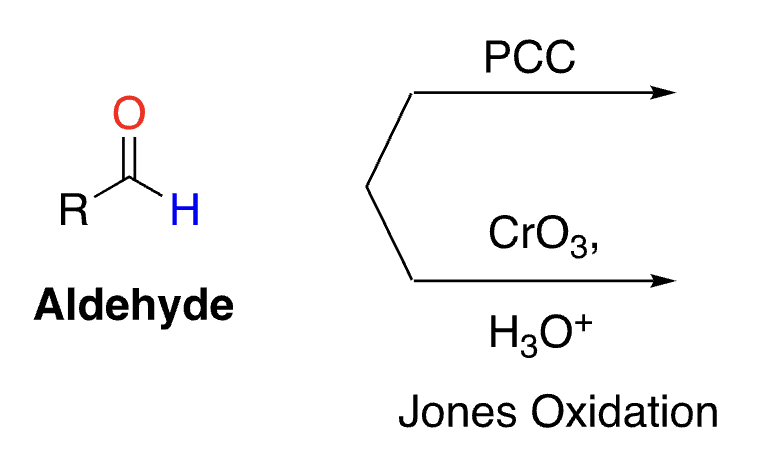
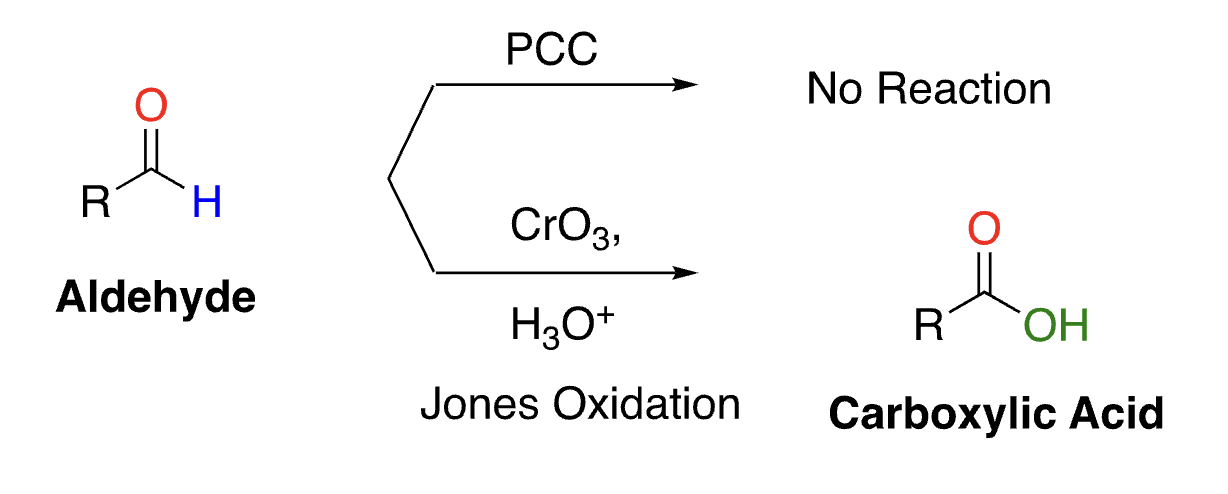
48
New cards
reacts with ketone
no reaction, does not oxidize
49
New cards
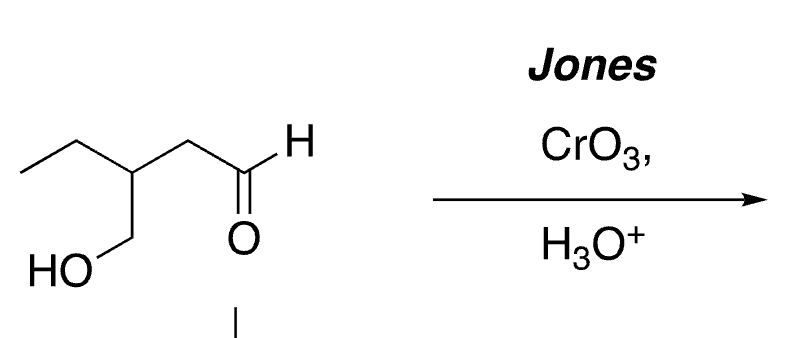
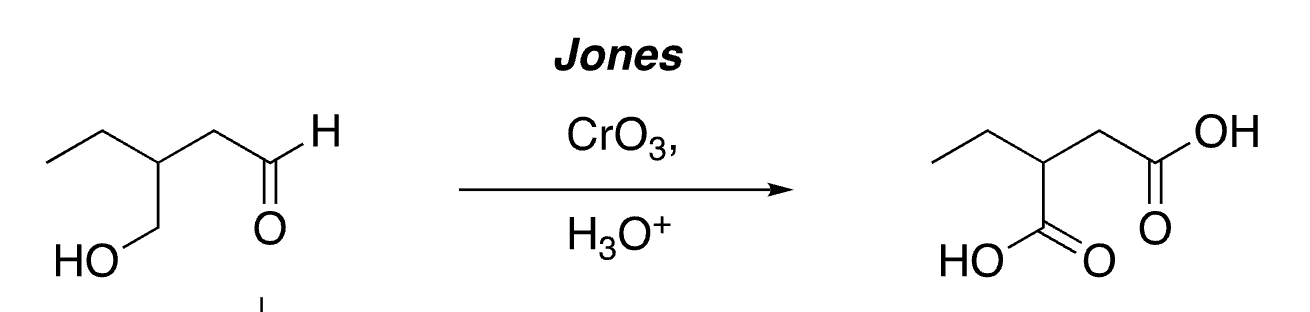
50
New cards
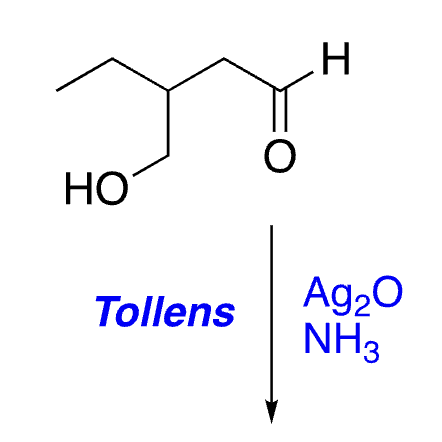
selective oxidation of aldehydes (not alcohols)
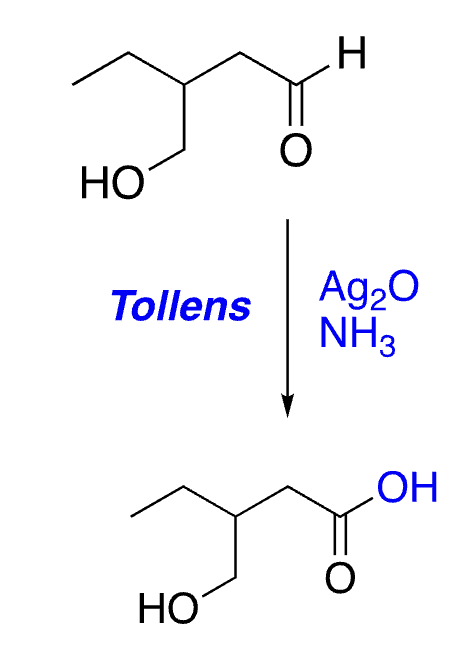
51
New cards
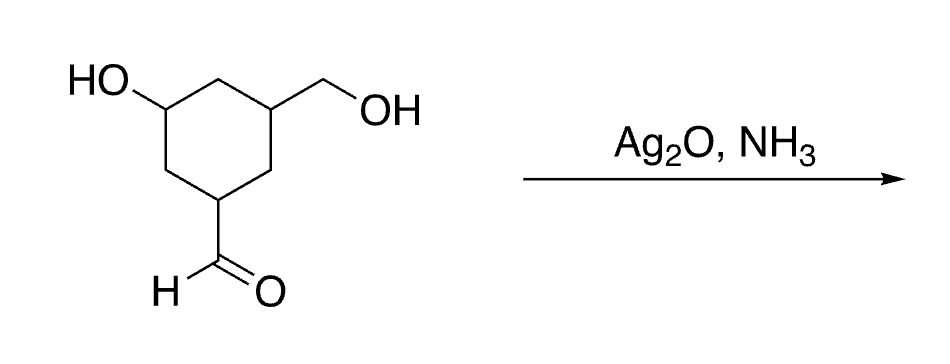
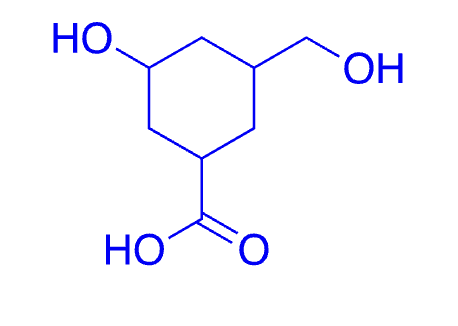
52
New cards
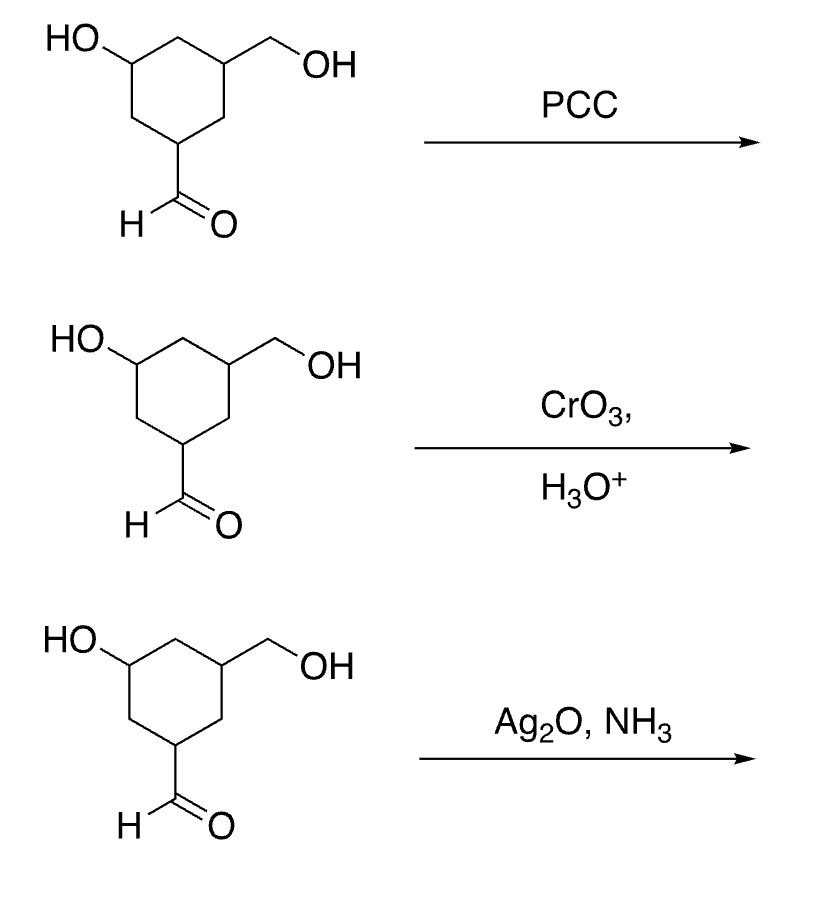
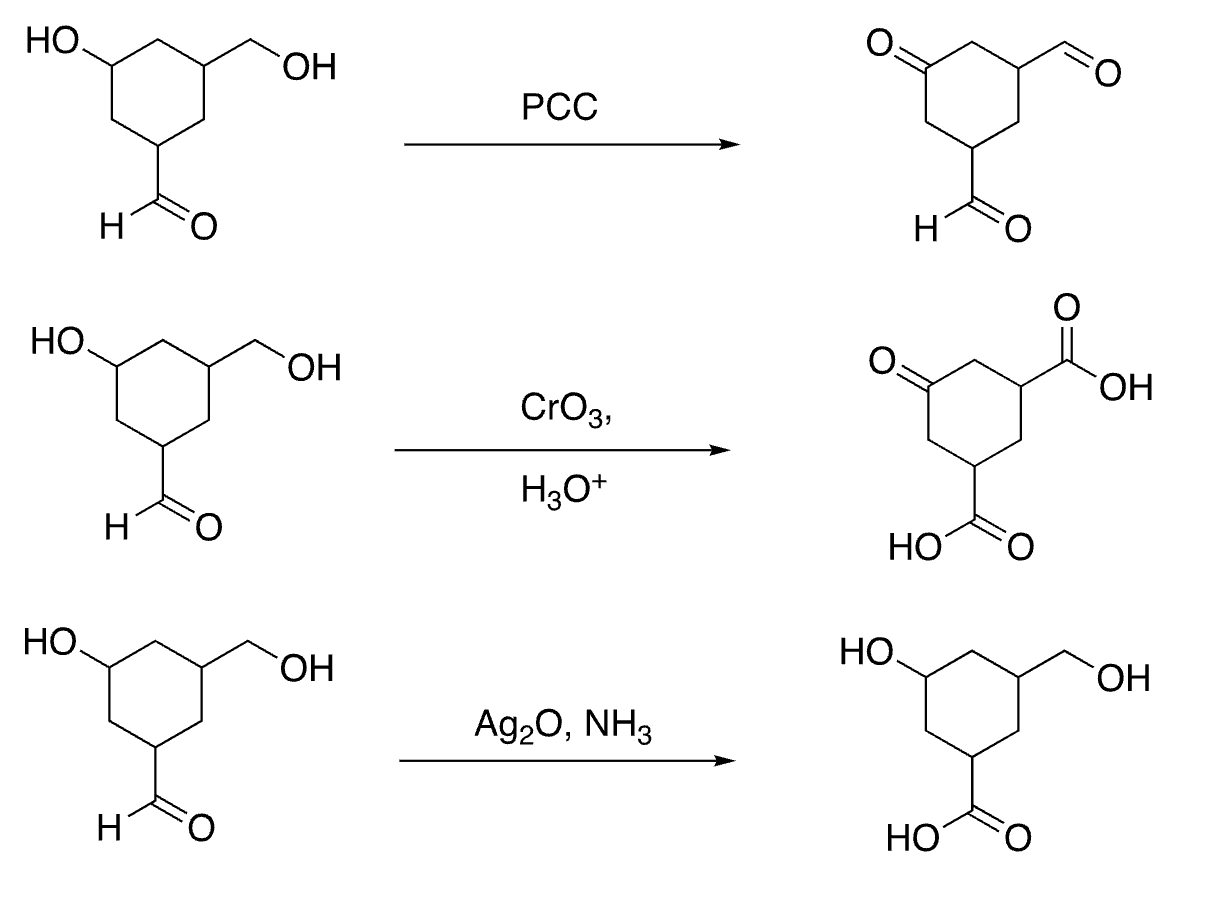
53
New cards
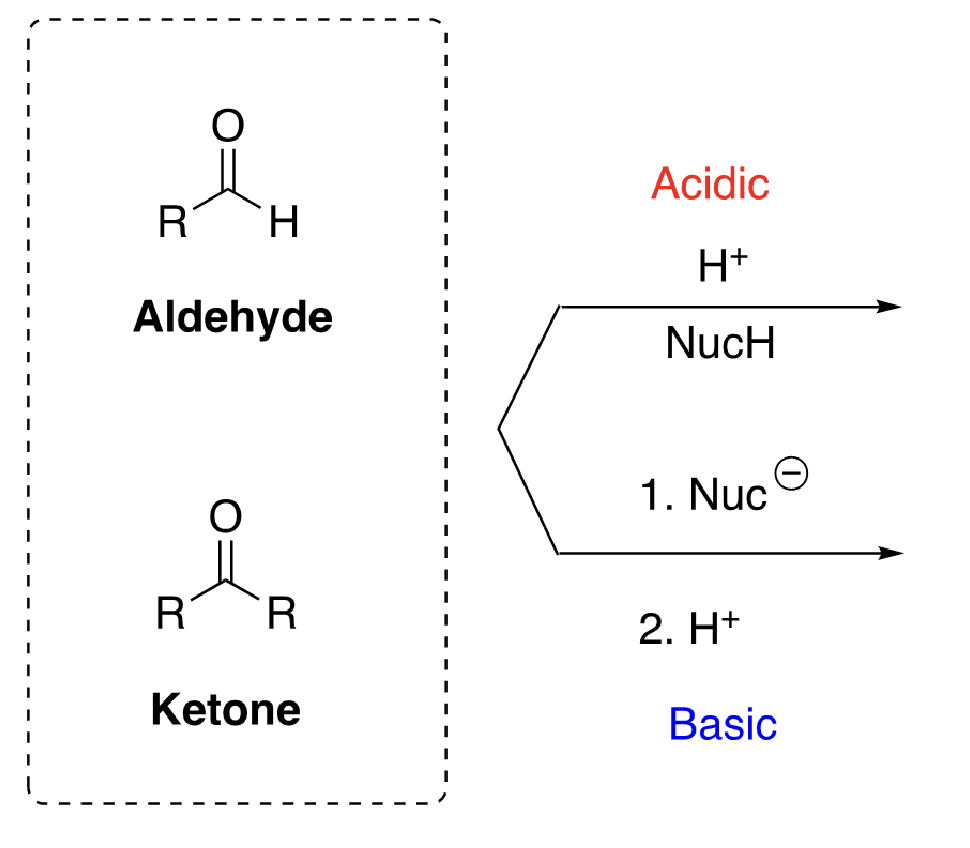
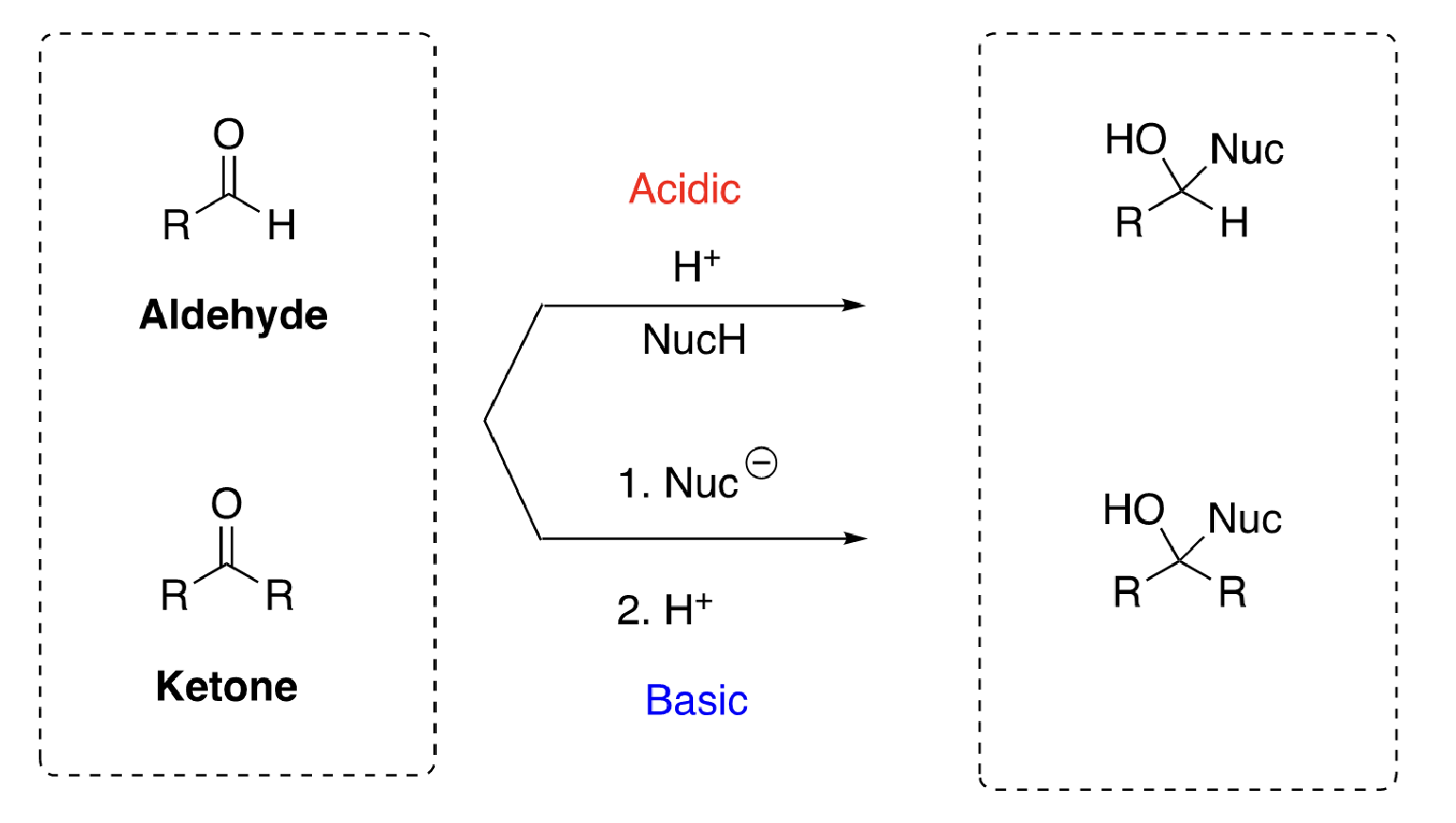
54
New cards
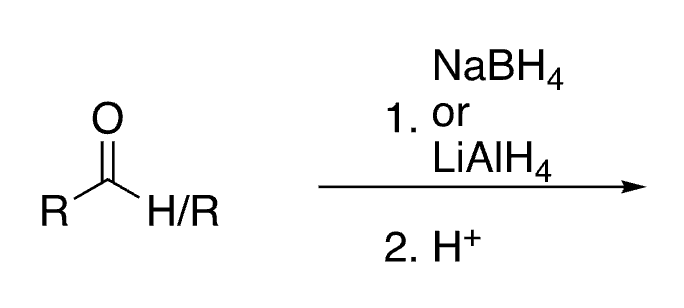
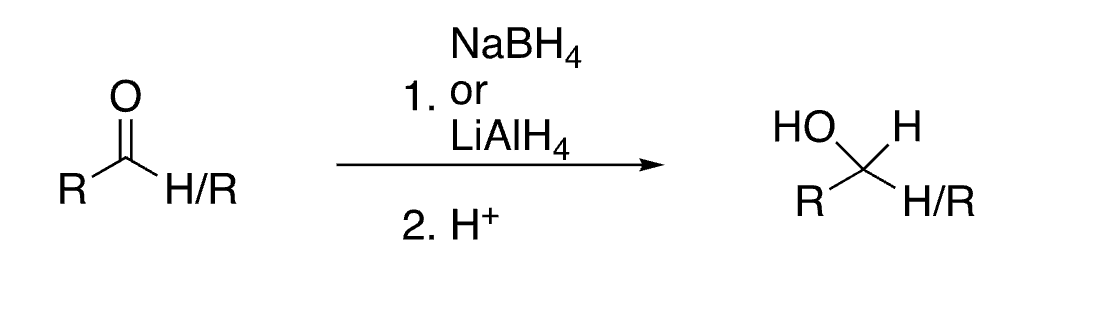
55
New cards
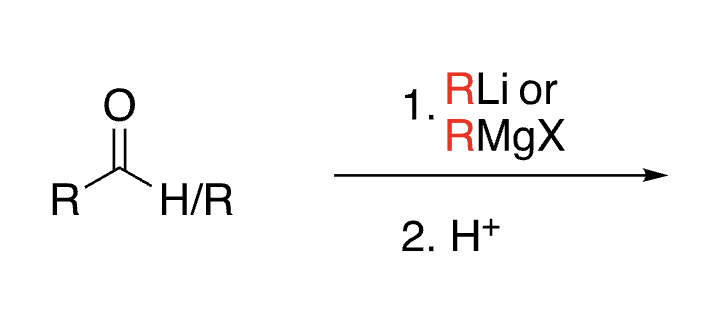
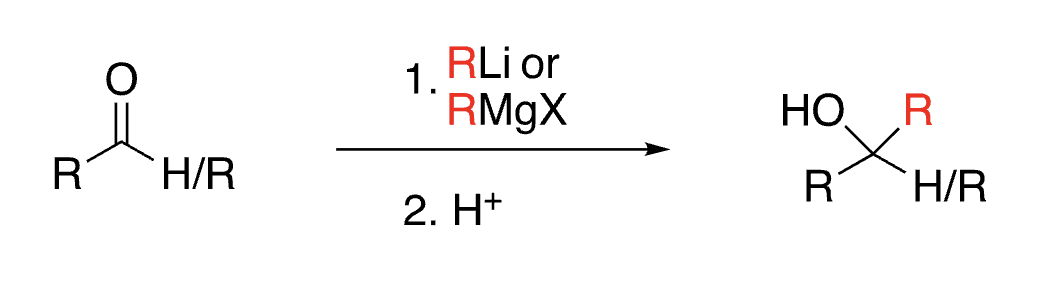
56
New cards
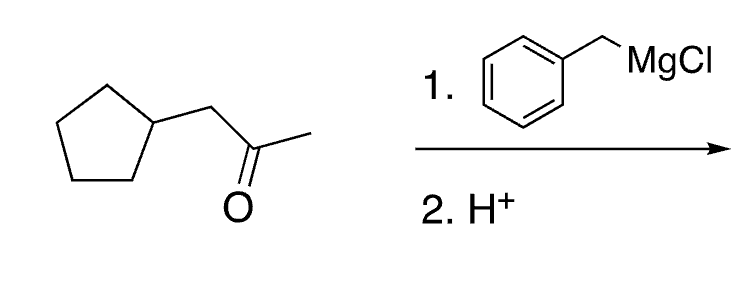
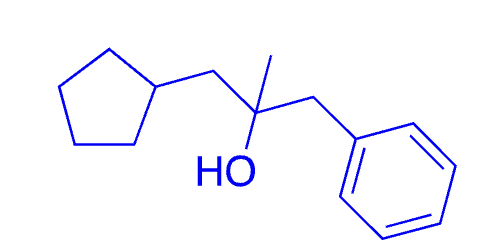
57
New cards
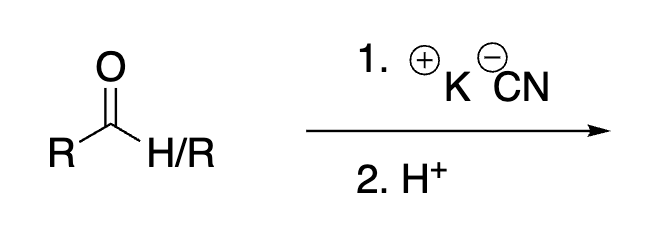
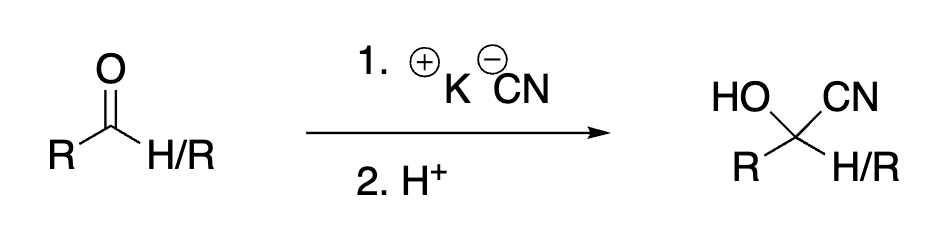
58
New cards
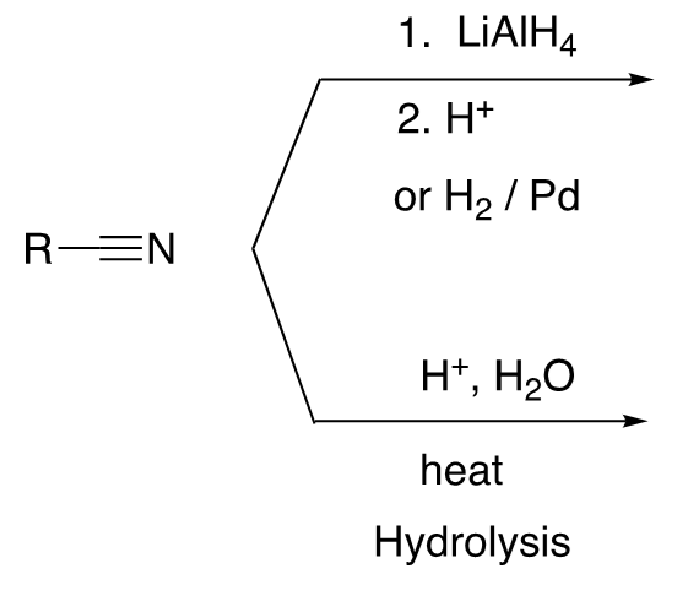
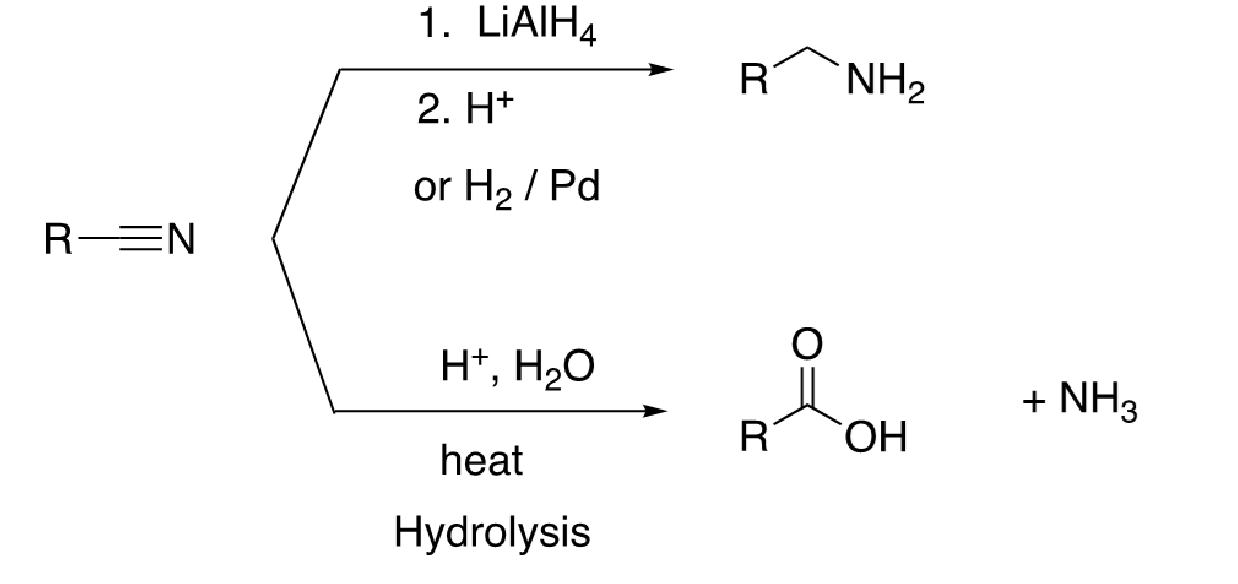
59
New cards
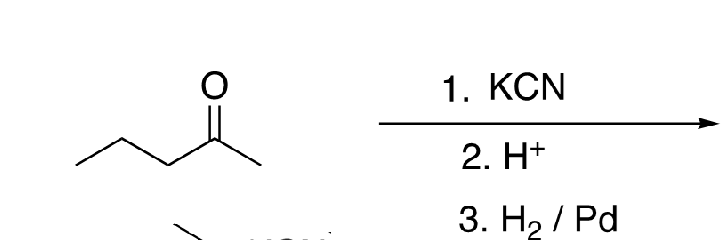
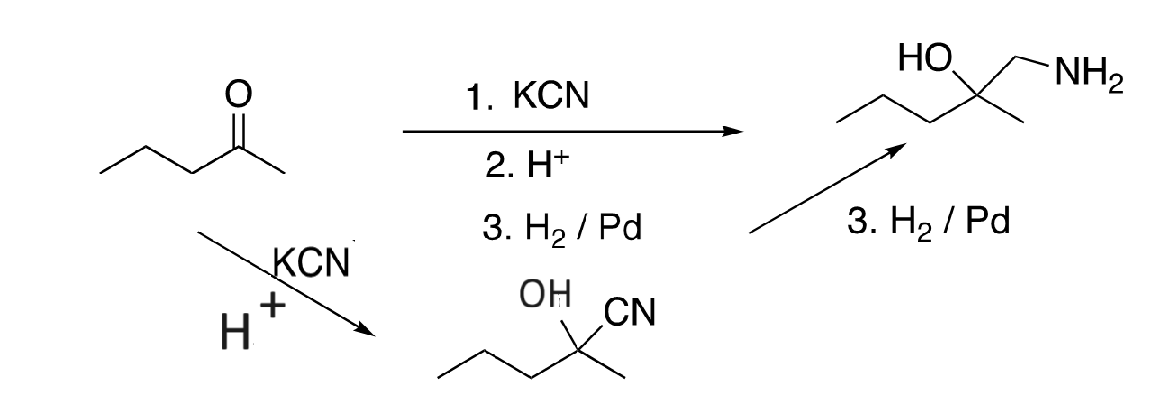
60
New cards
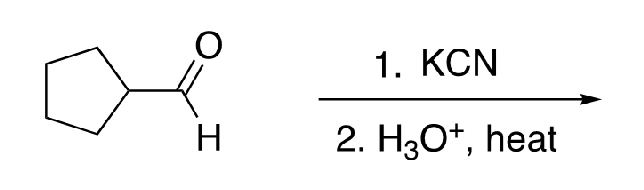
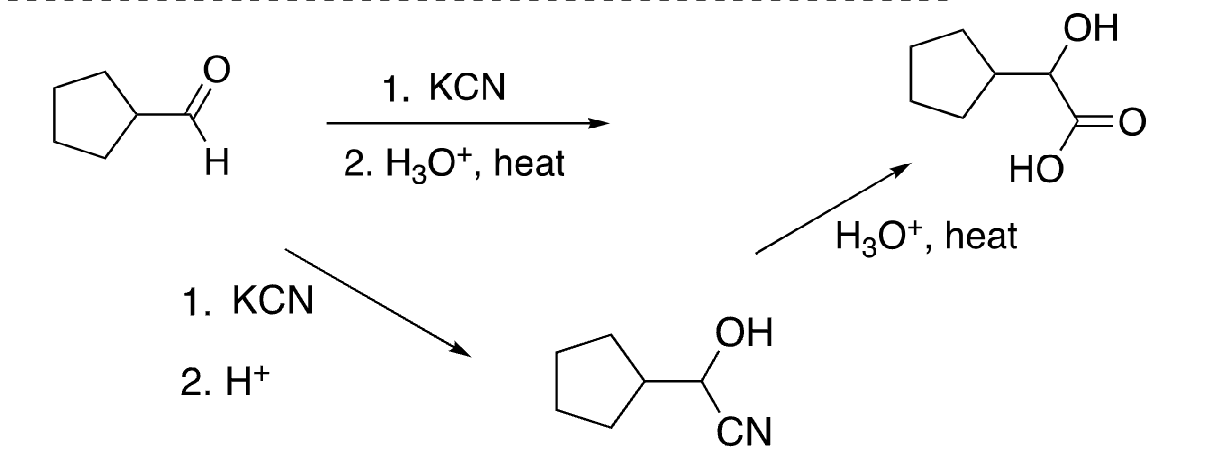
61
New cards
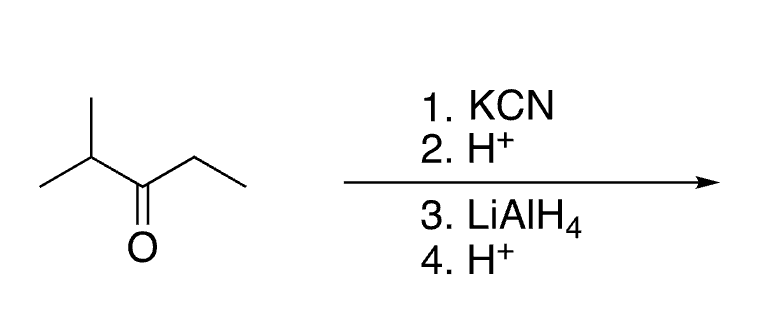
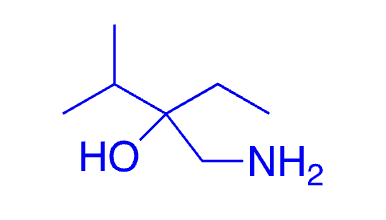
62
New cards
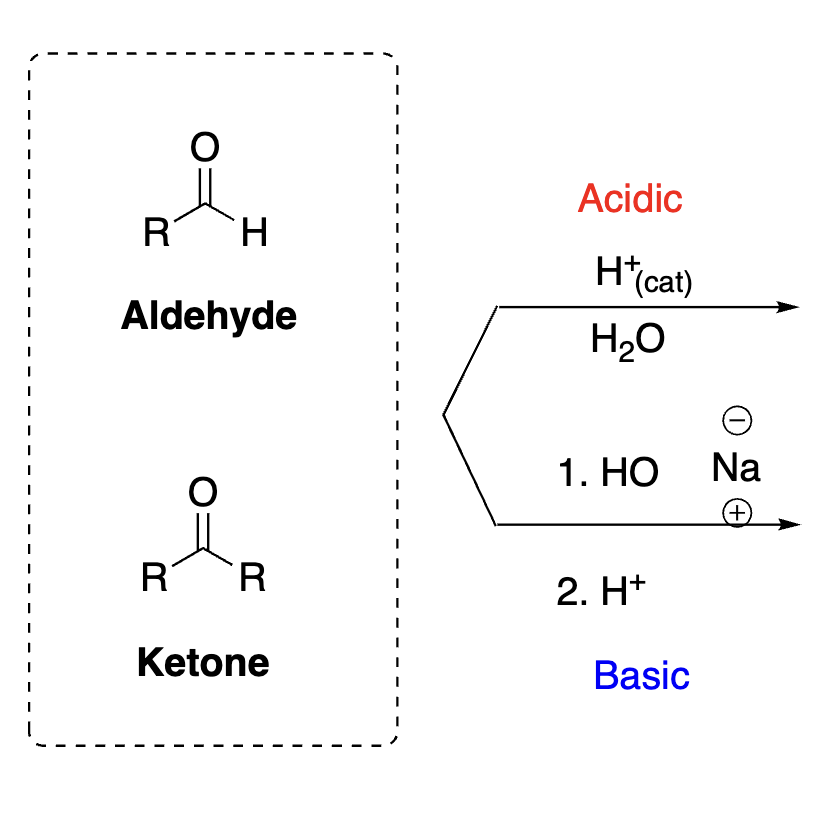
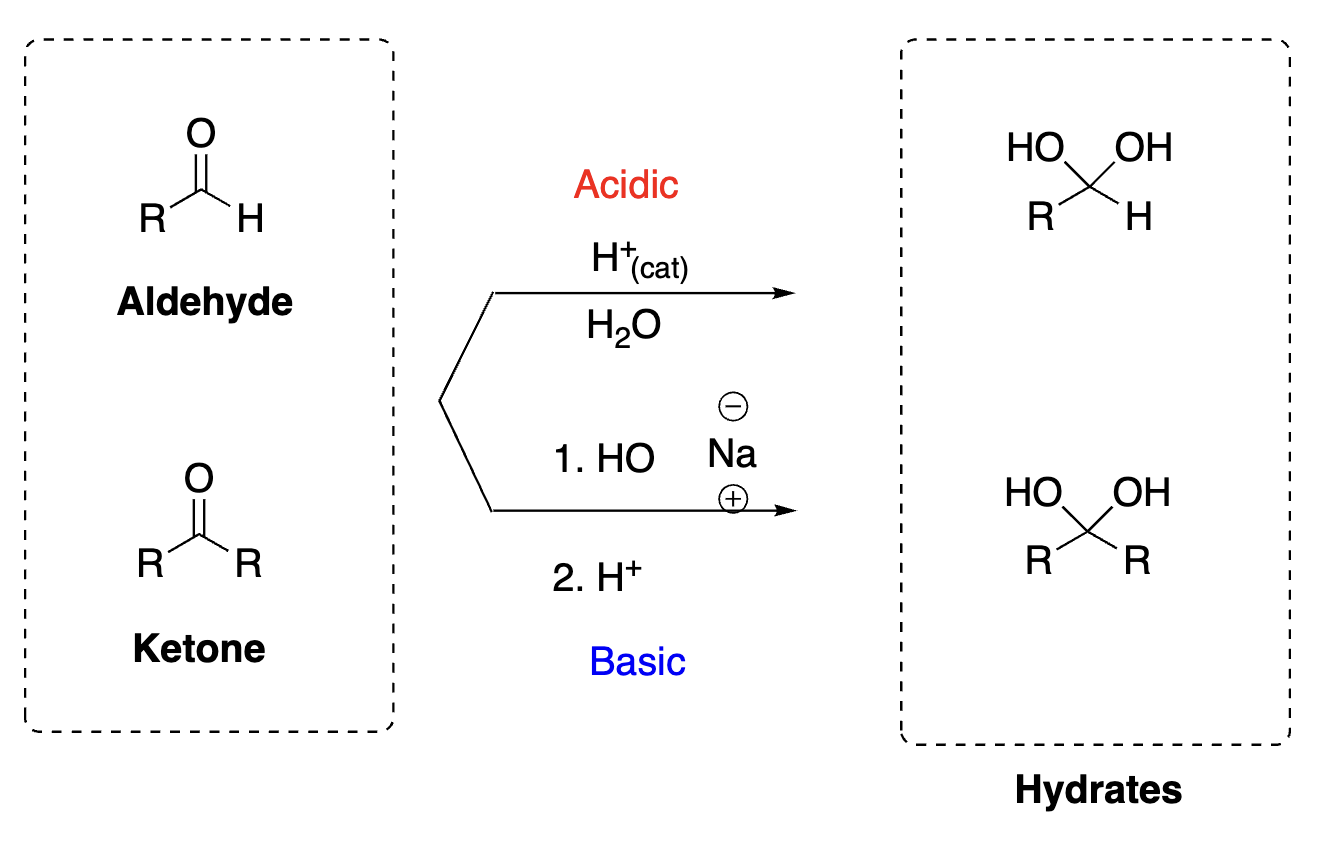
63
New cards
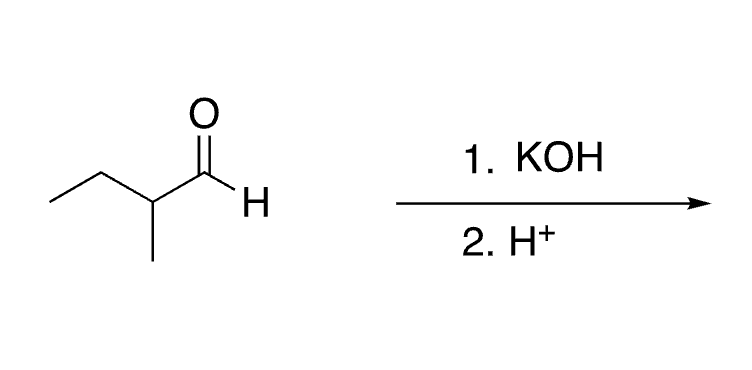
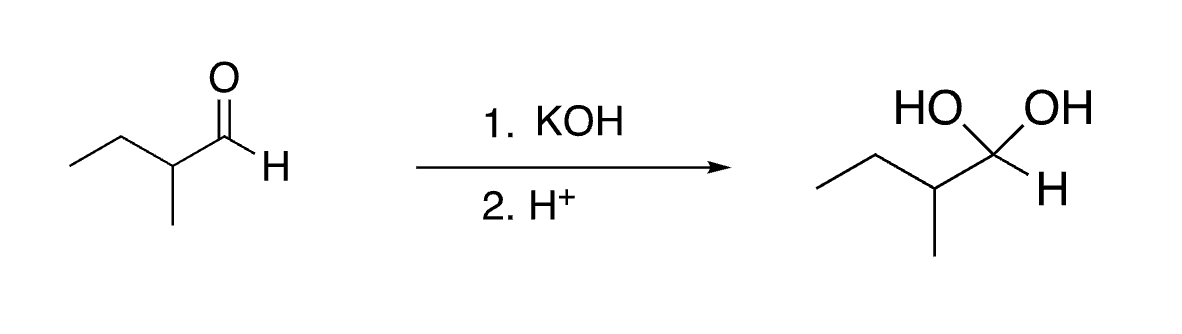
64
New cards
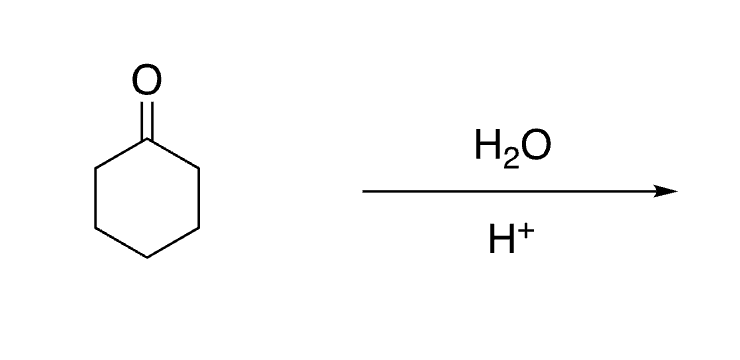
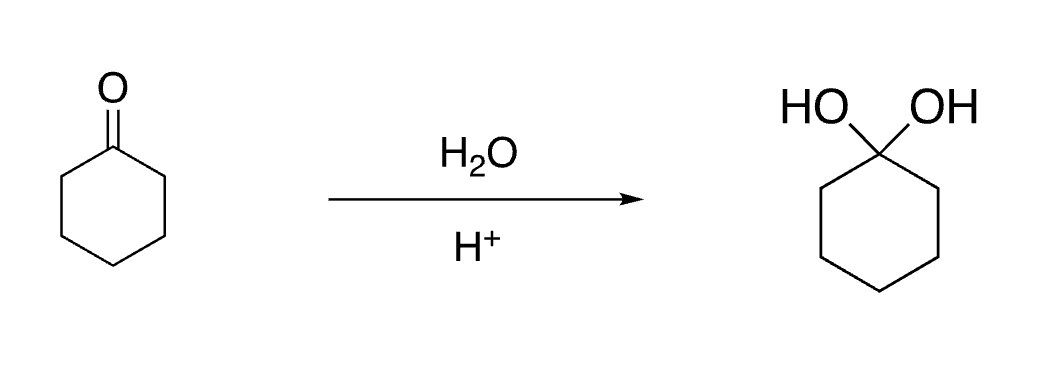
65
New cards
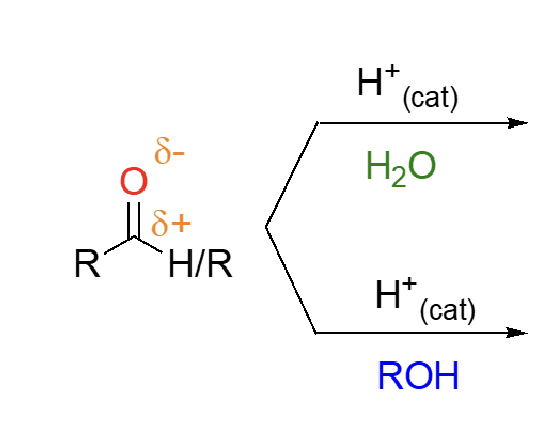
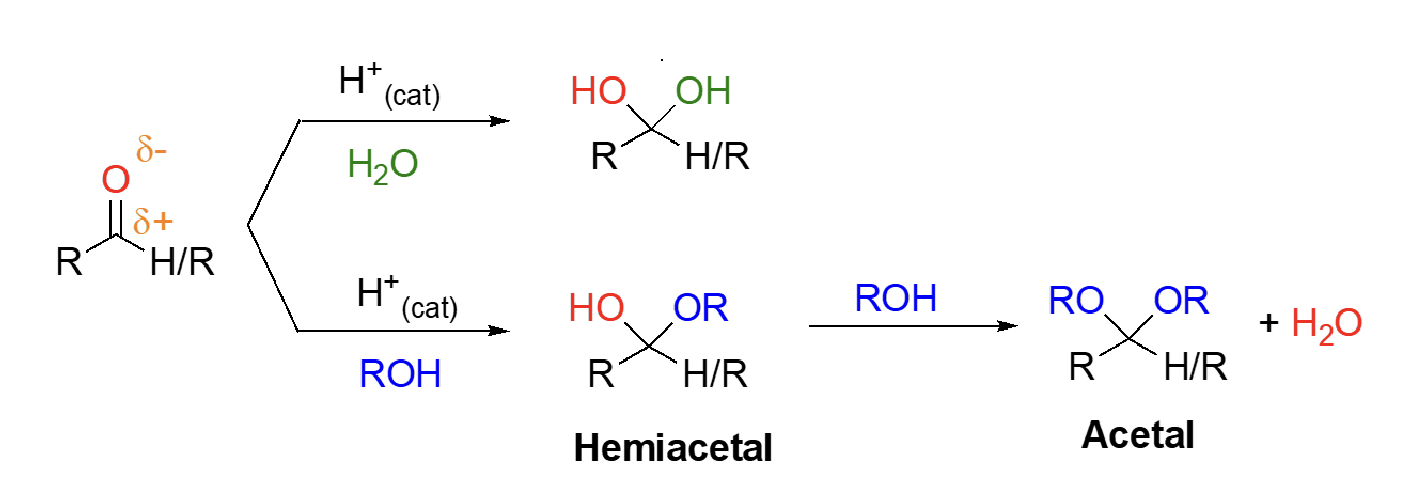
66
New cards
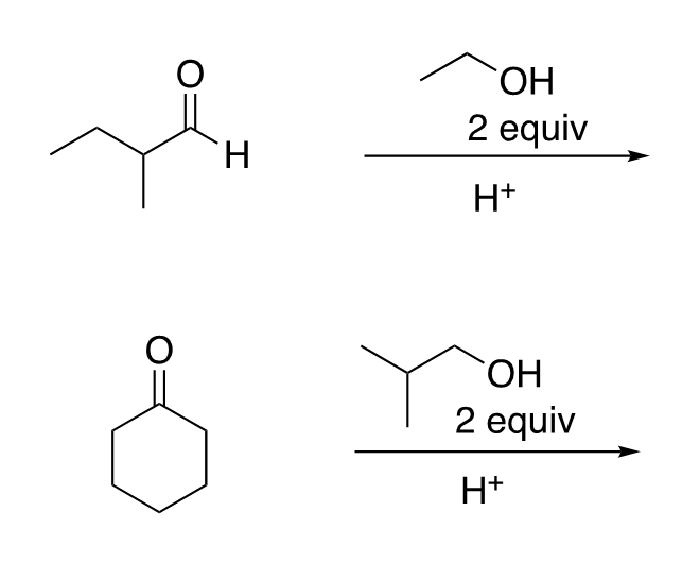
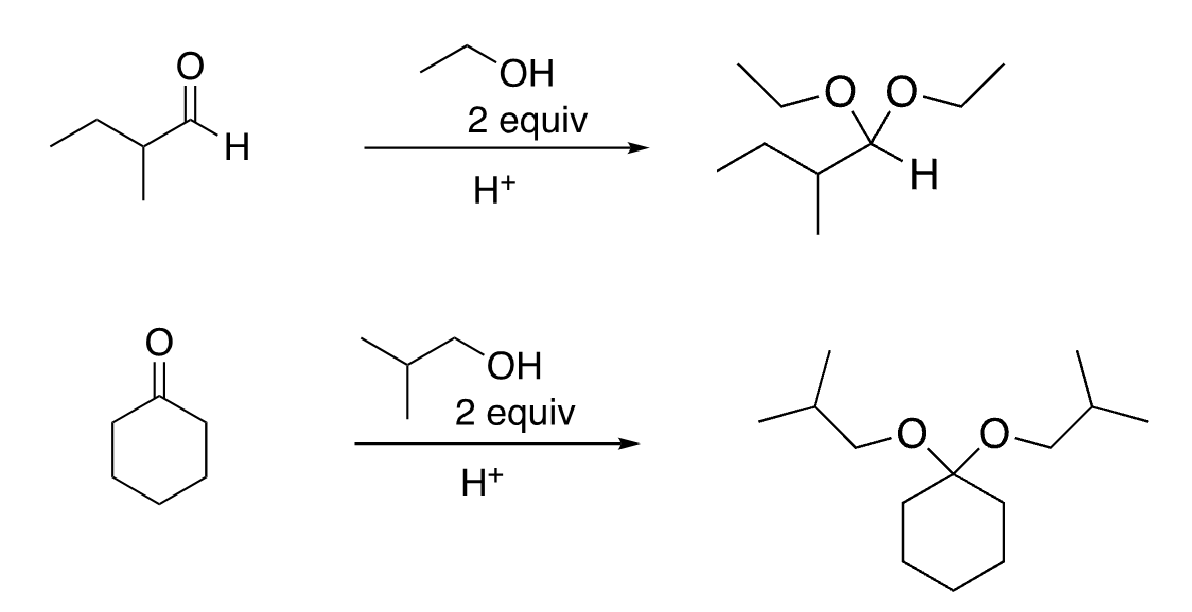
67
New cards
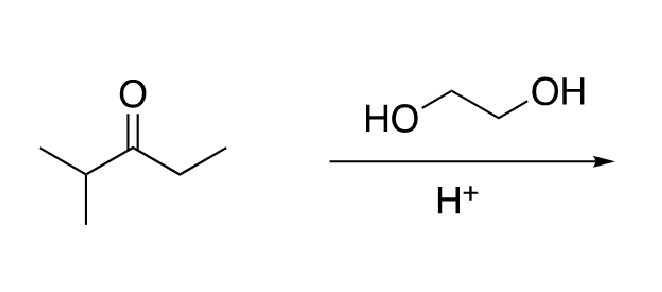
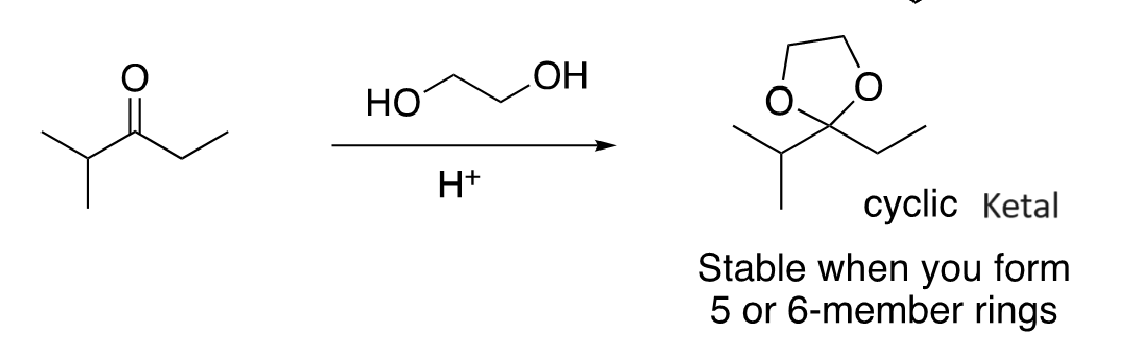
68
New cards
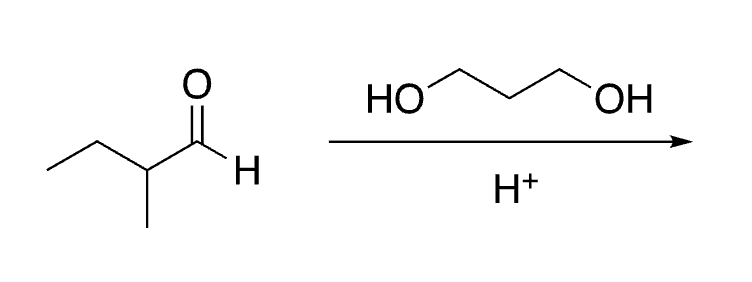
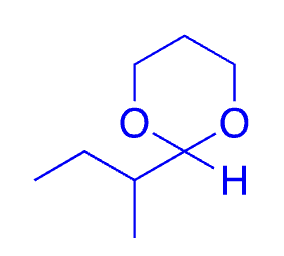
69
New cards
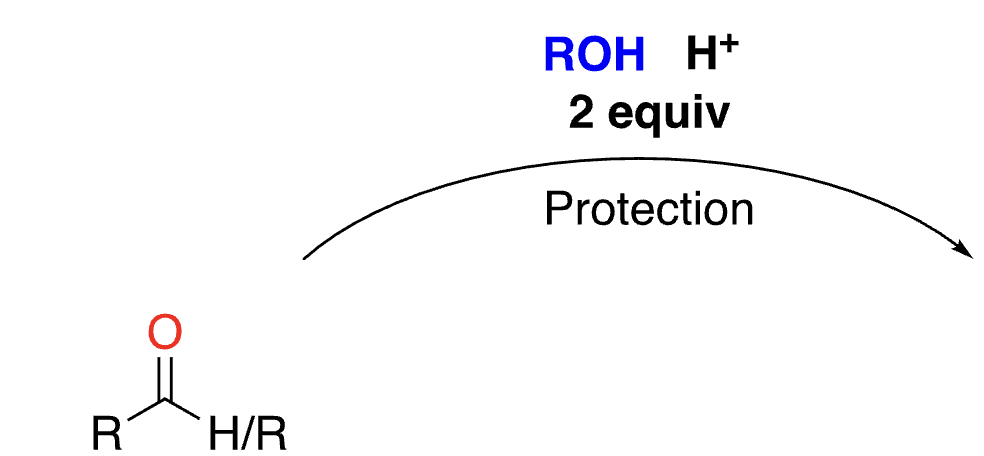
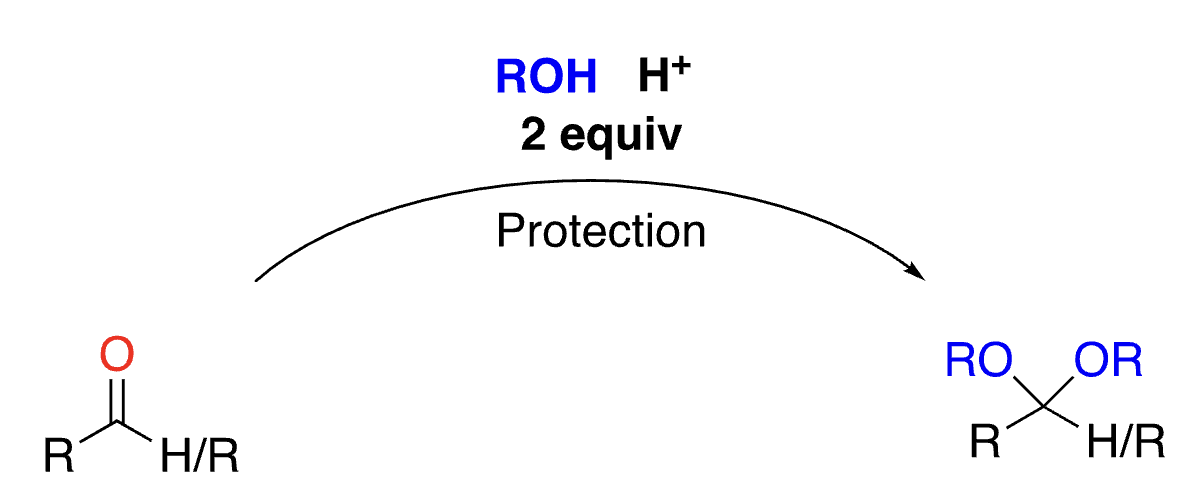
70
New cards
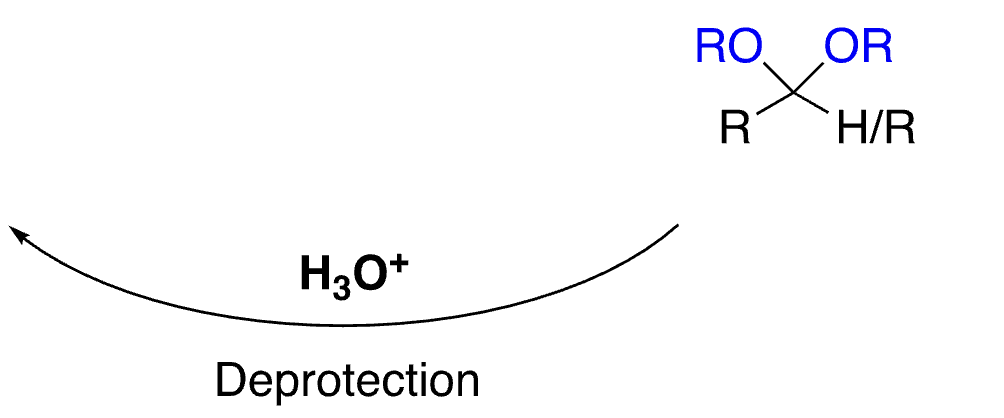
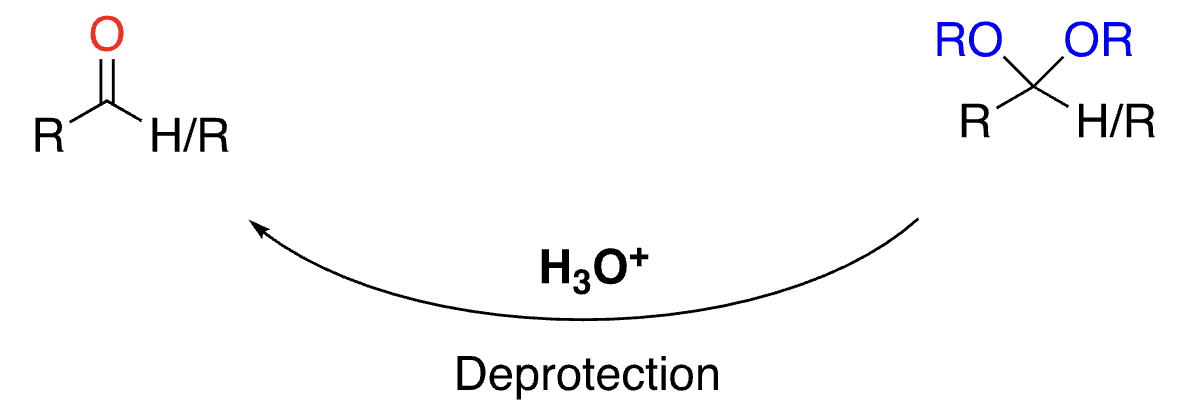
71
New cards
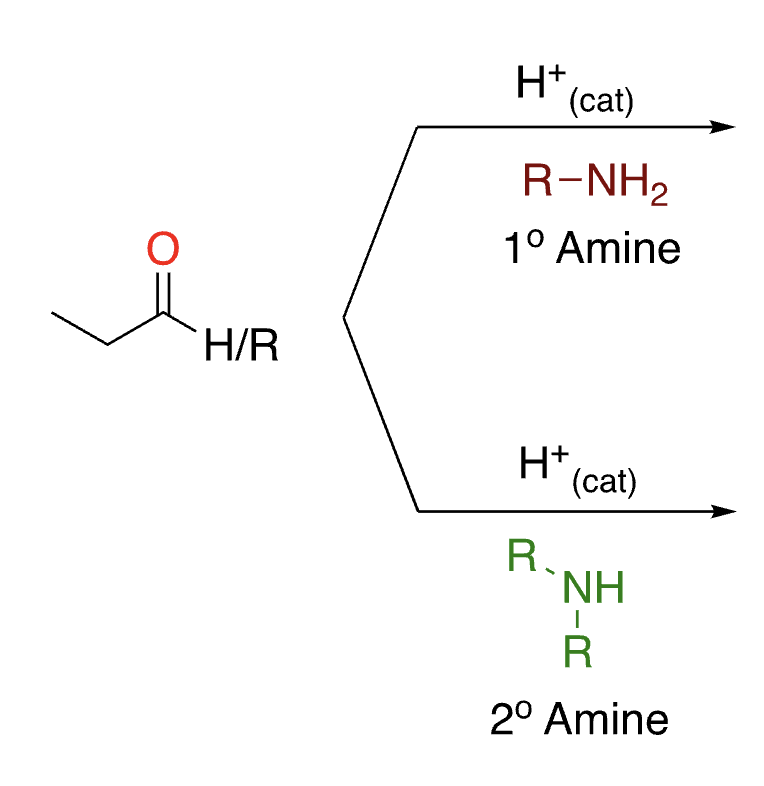
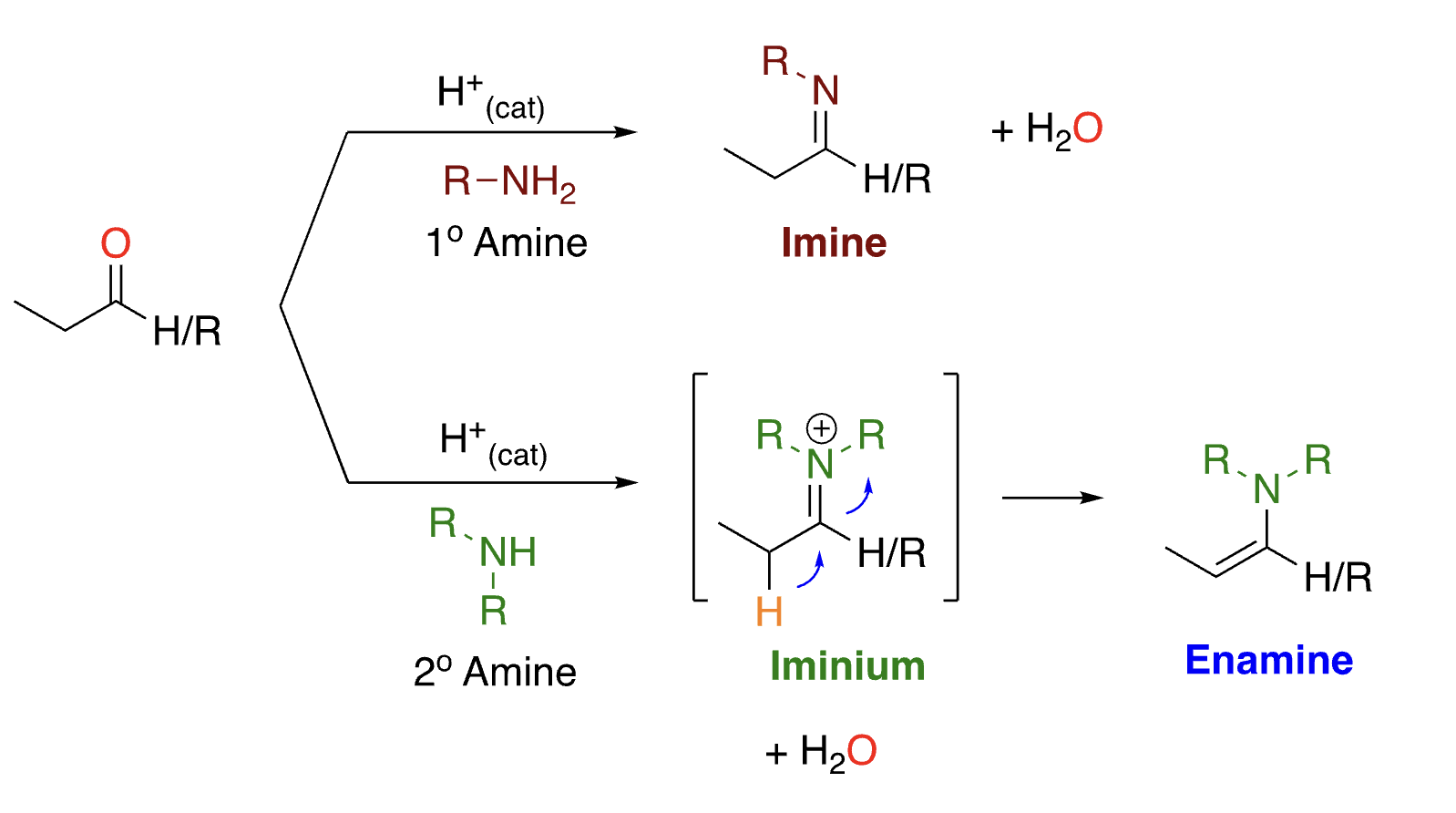
72
New cards
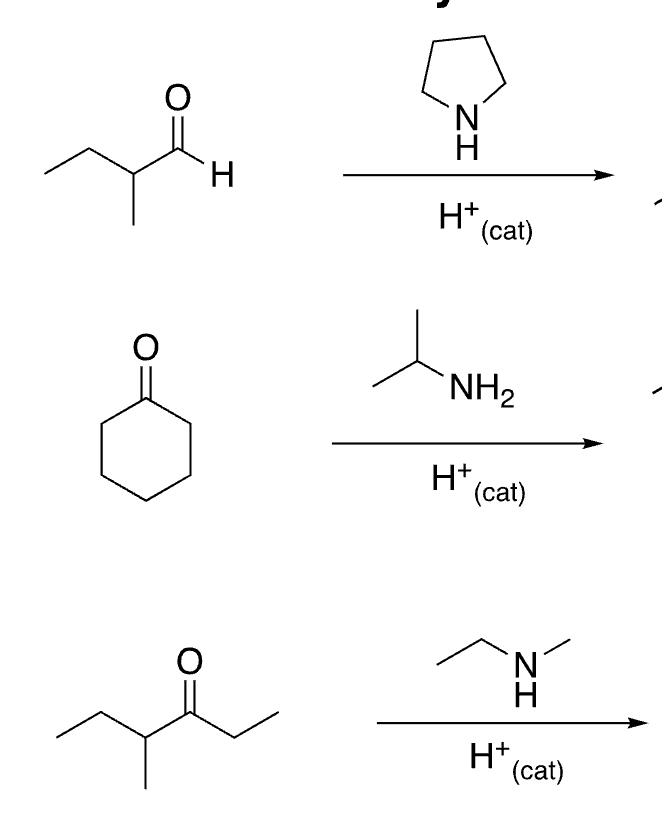
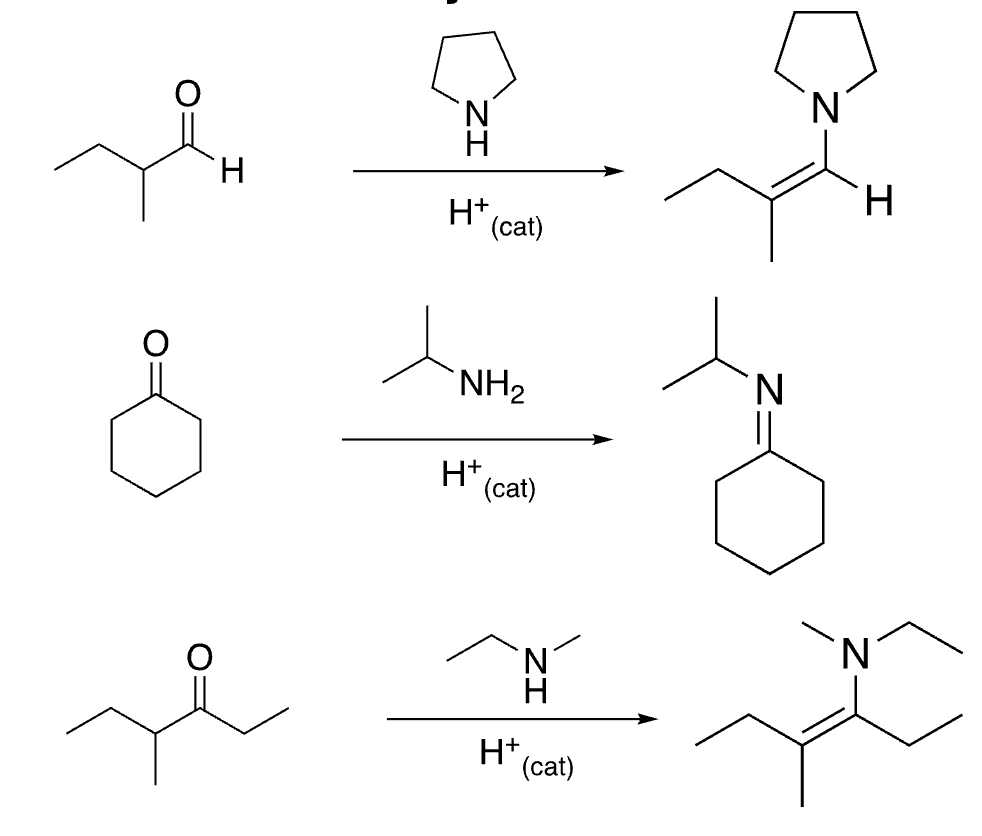
73
New cards
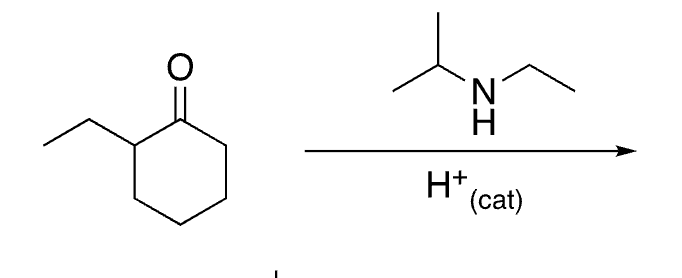
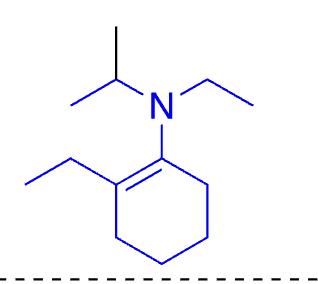
74
New cards
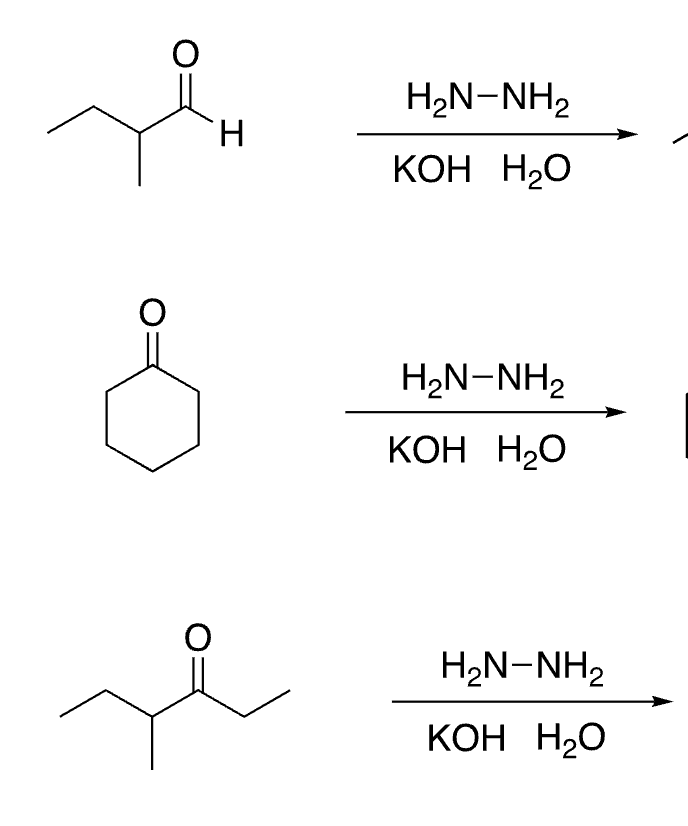
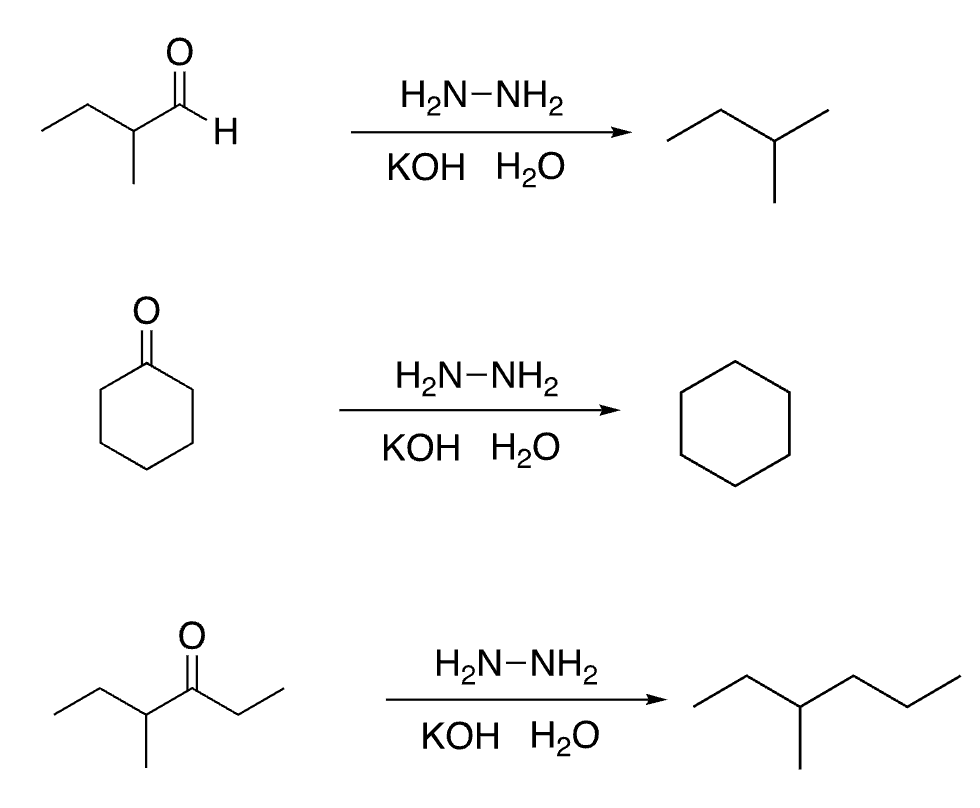
75
New cards
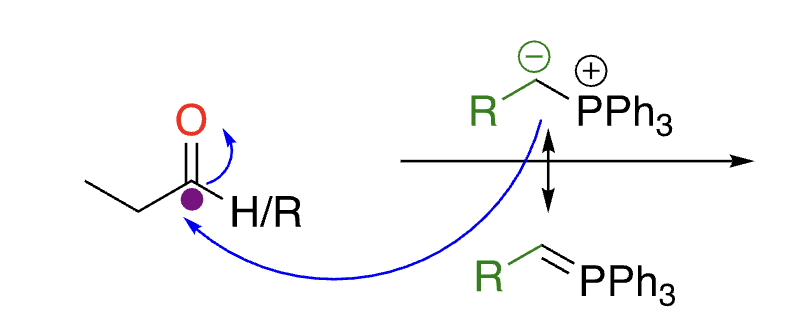
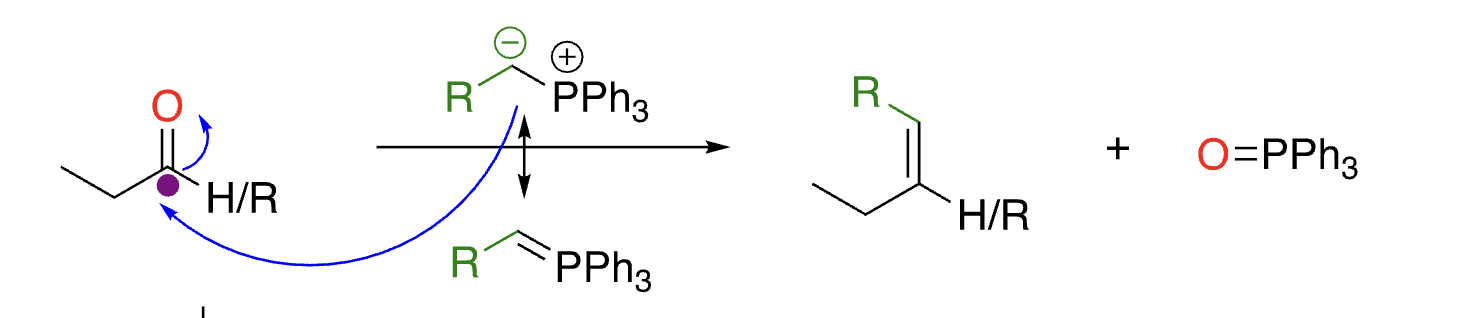
76
New cards
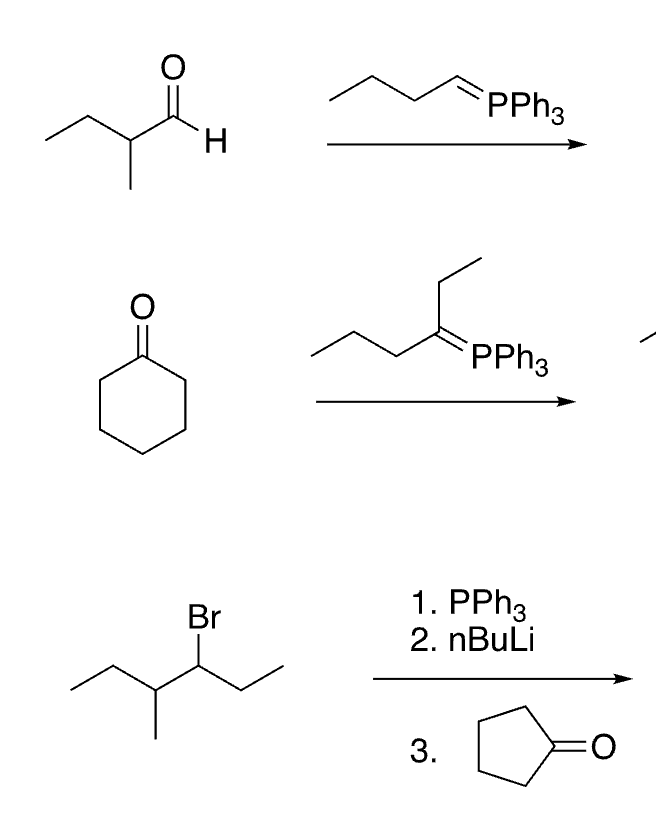
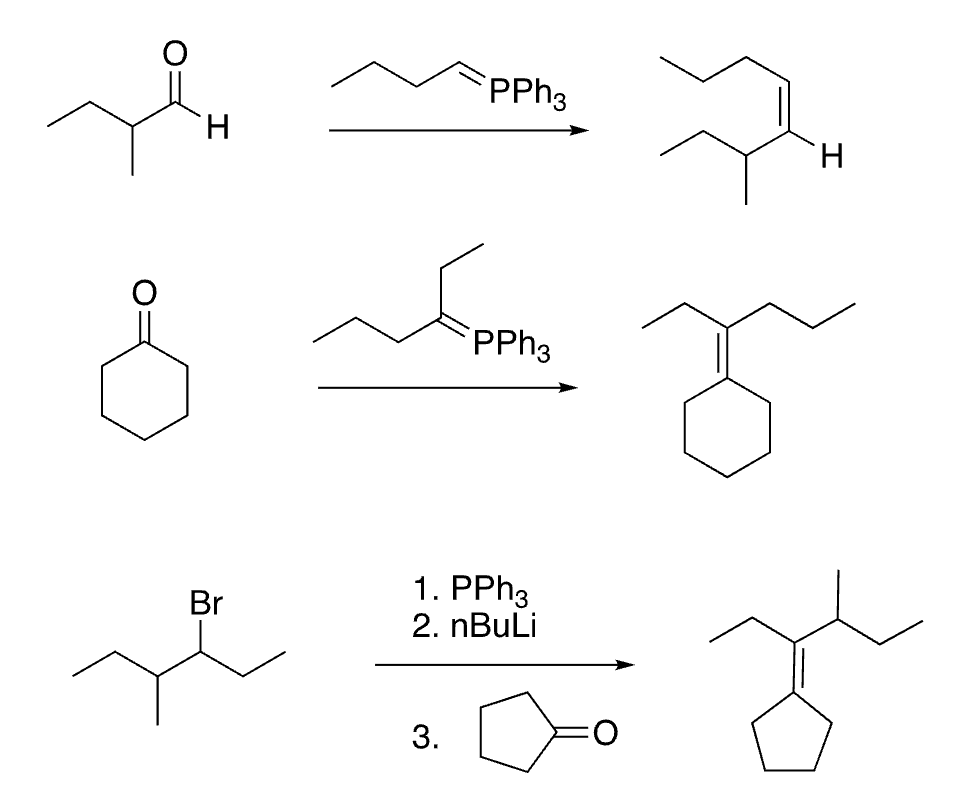
77
New cards
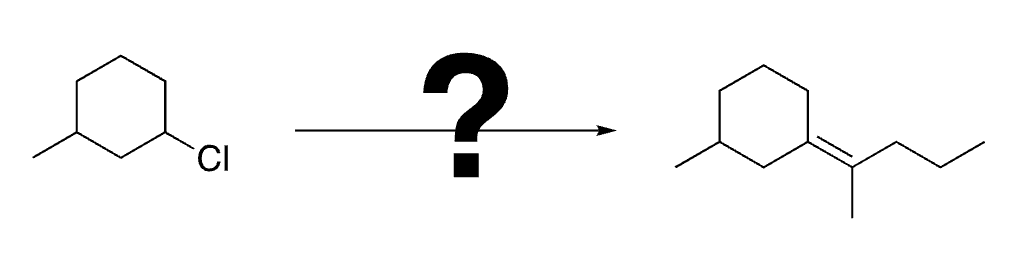
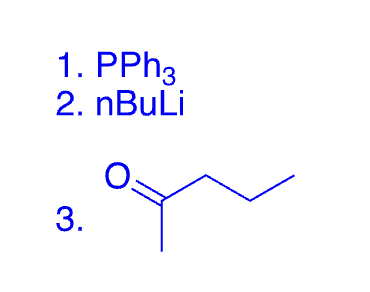
78
New cards
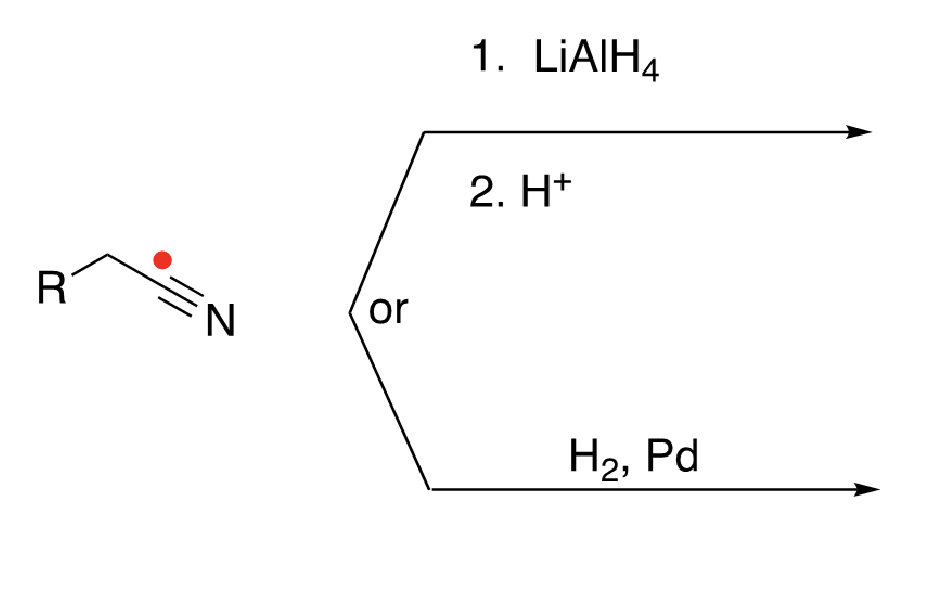
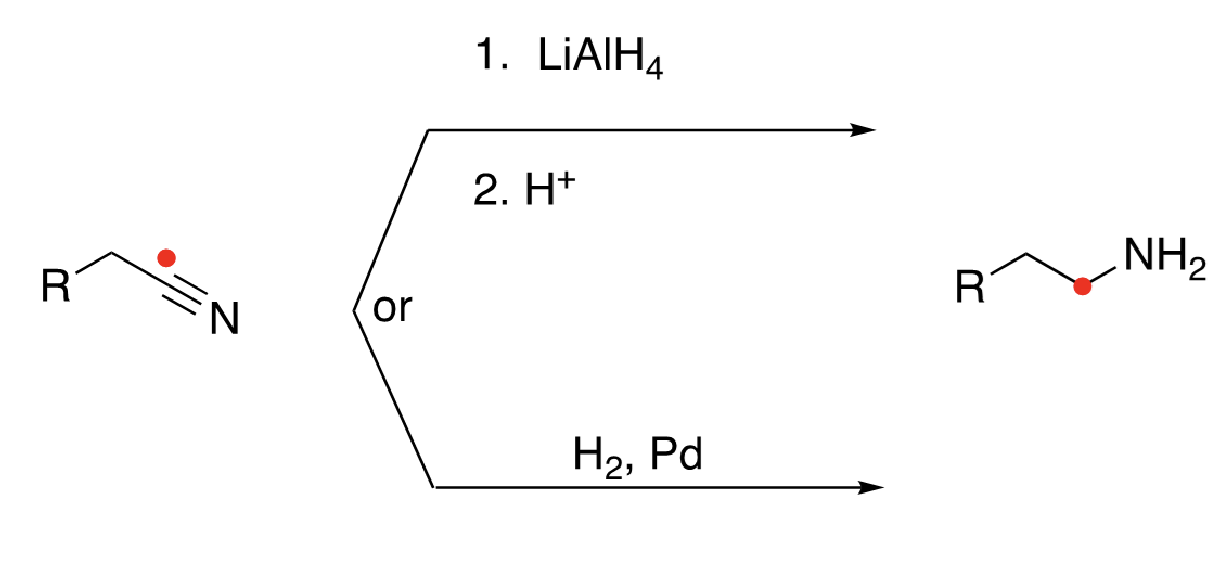
79
New cards
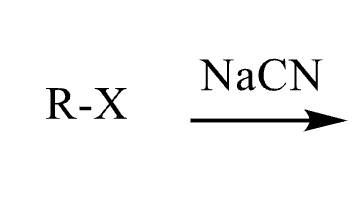
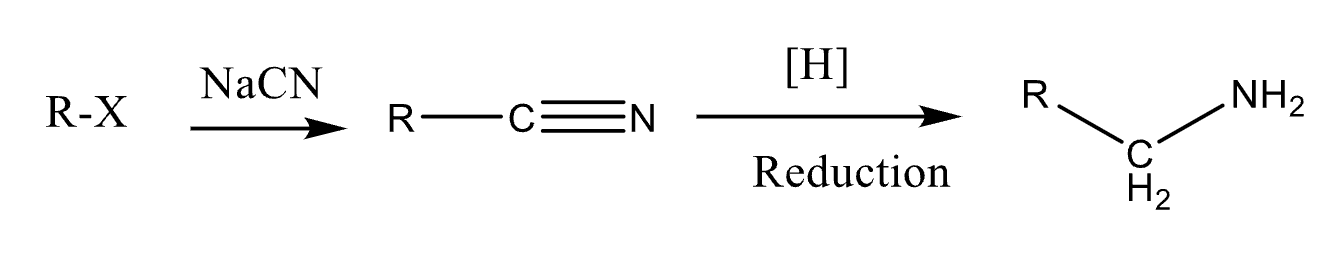
80
New cards
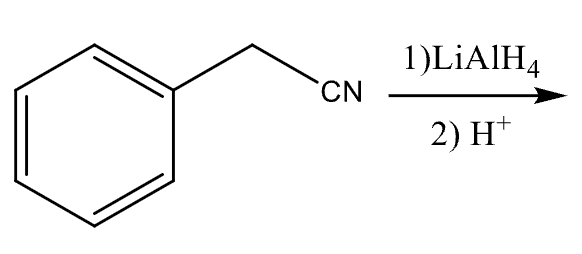
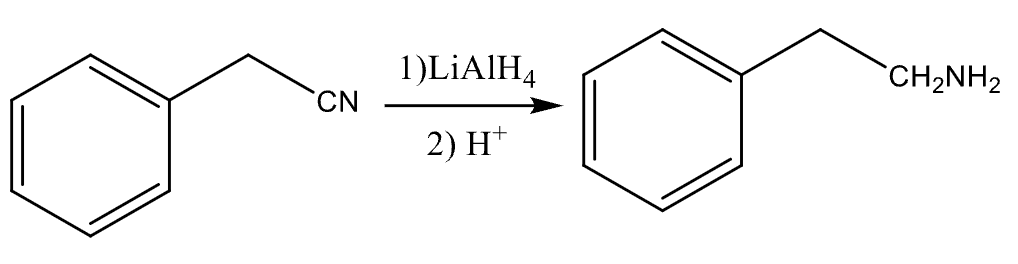
81
New cards
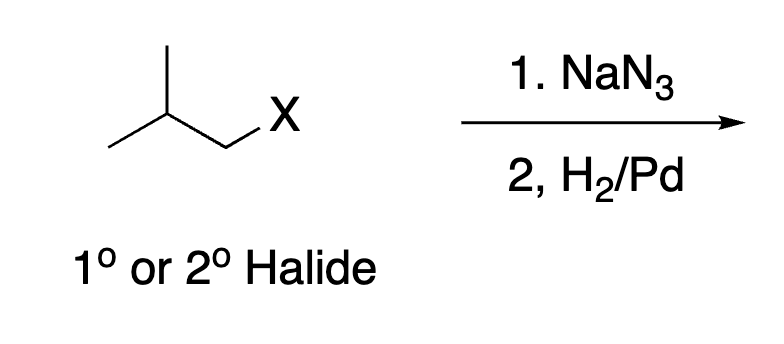
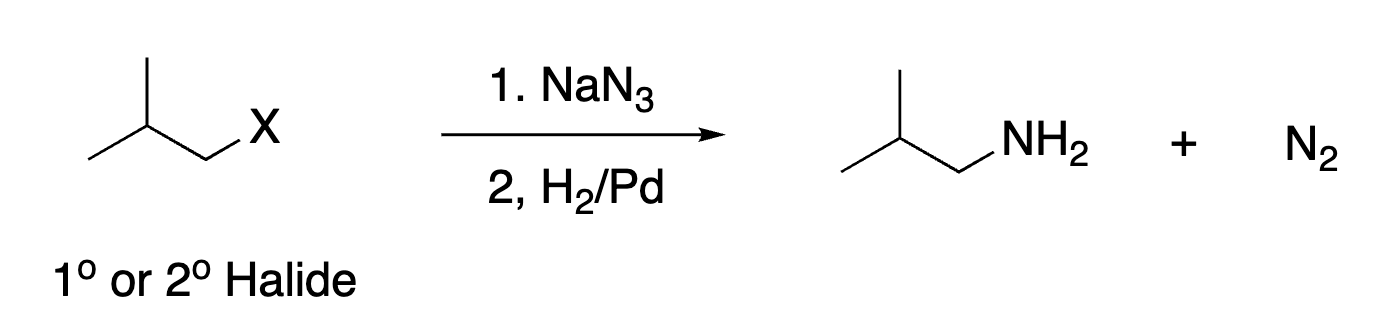
82
New cards
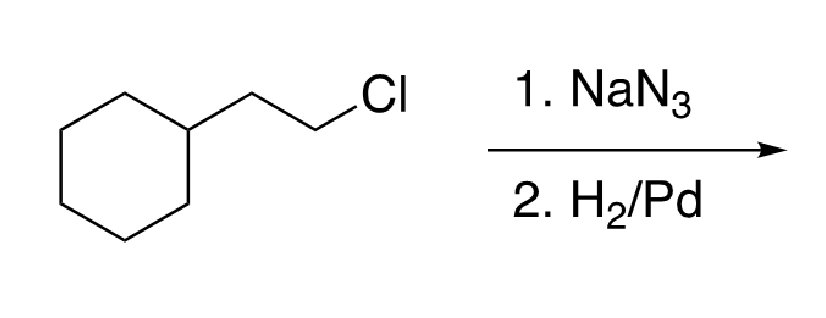
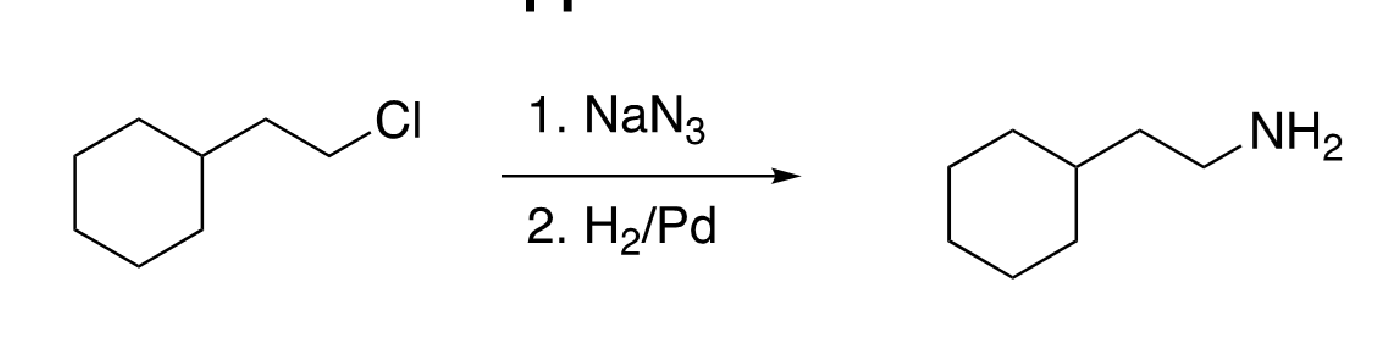
83
New cards
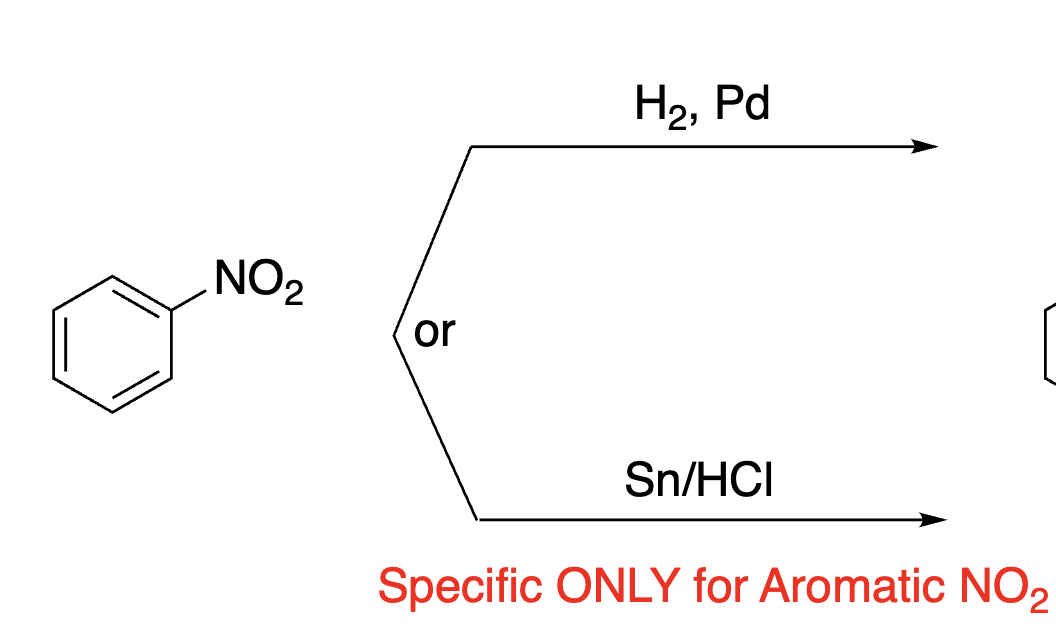
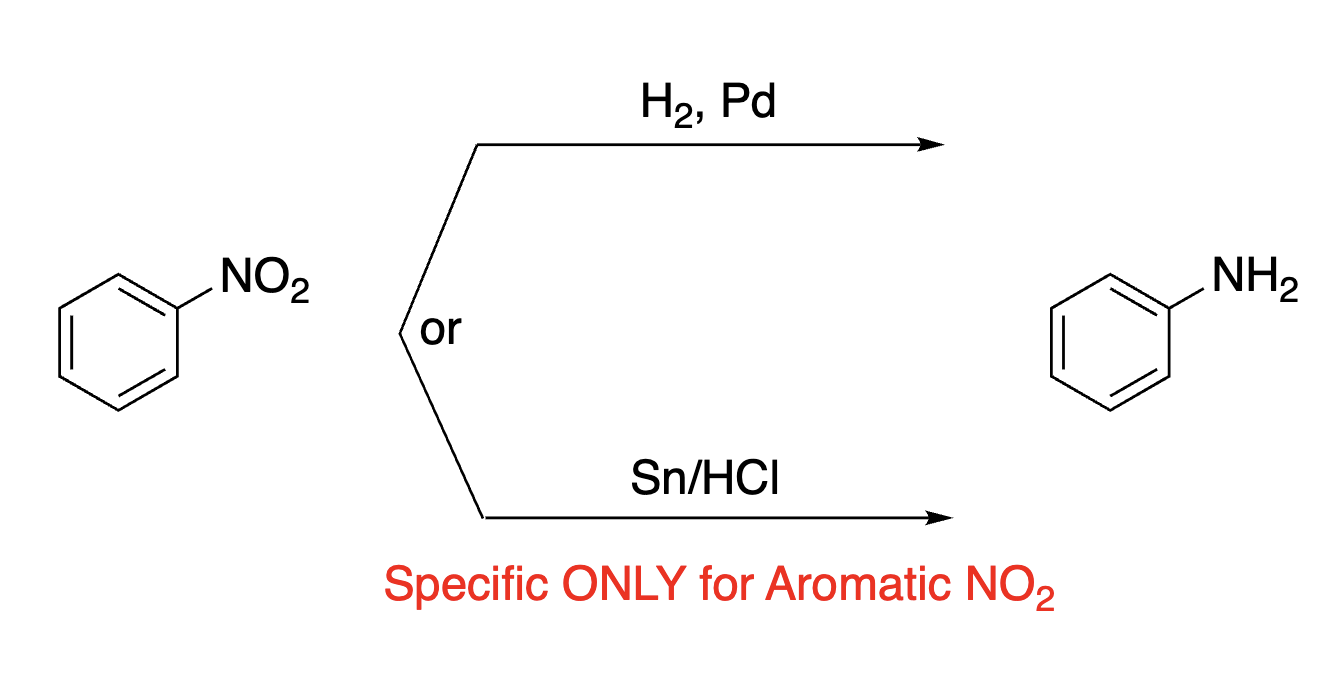
84
New cards
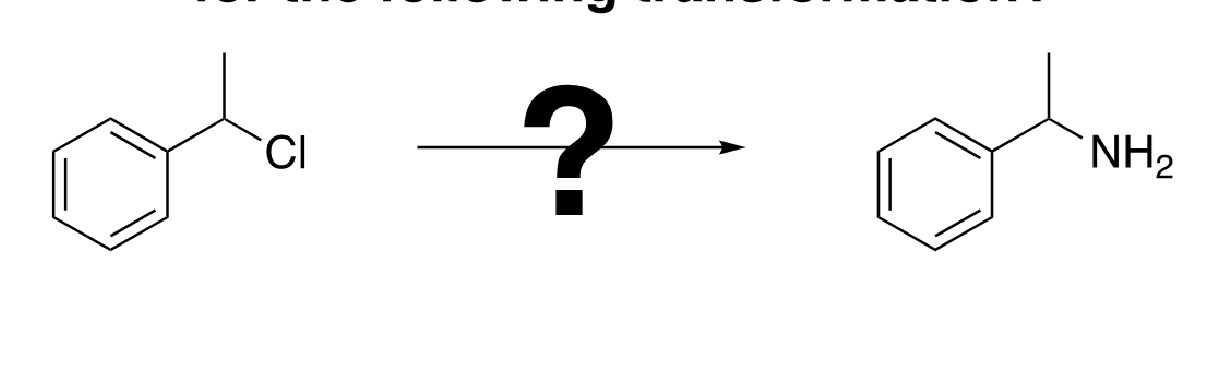
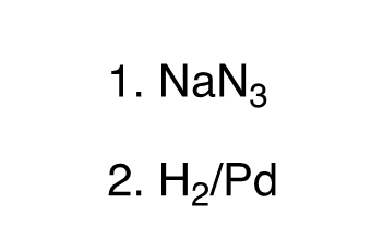
85
New cards
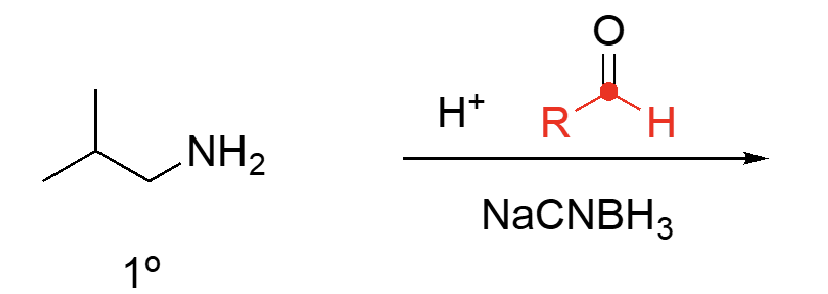
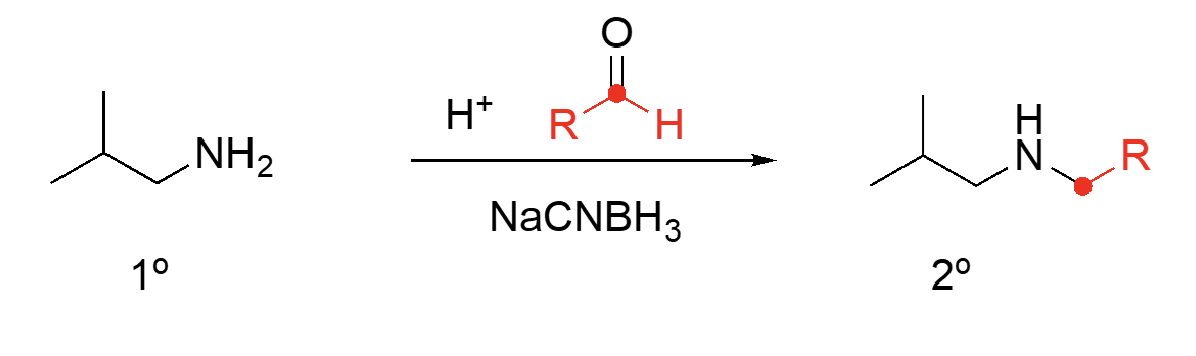
86
New cards
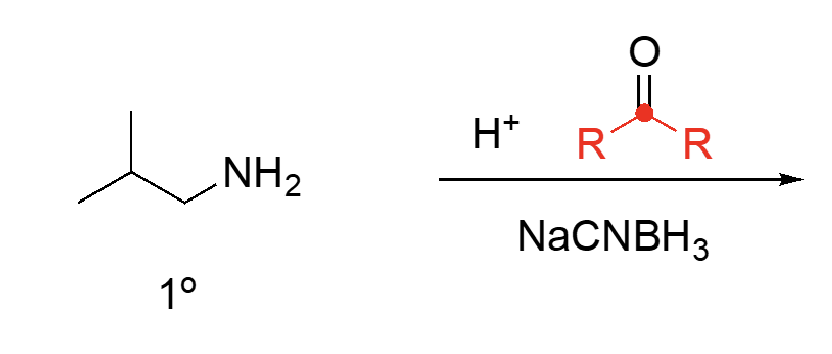
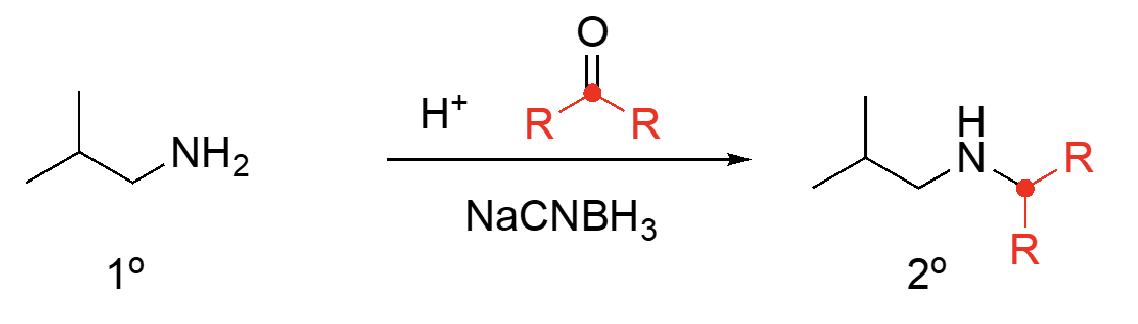
87
New cards
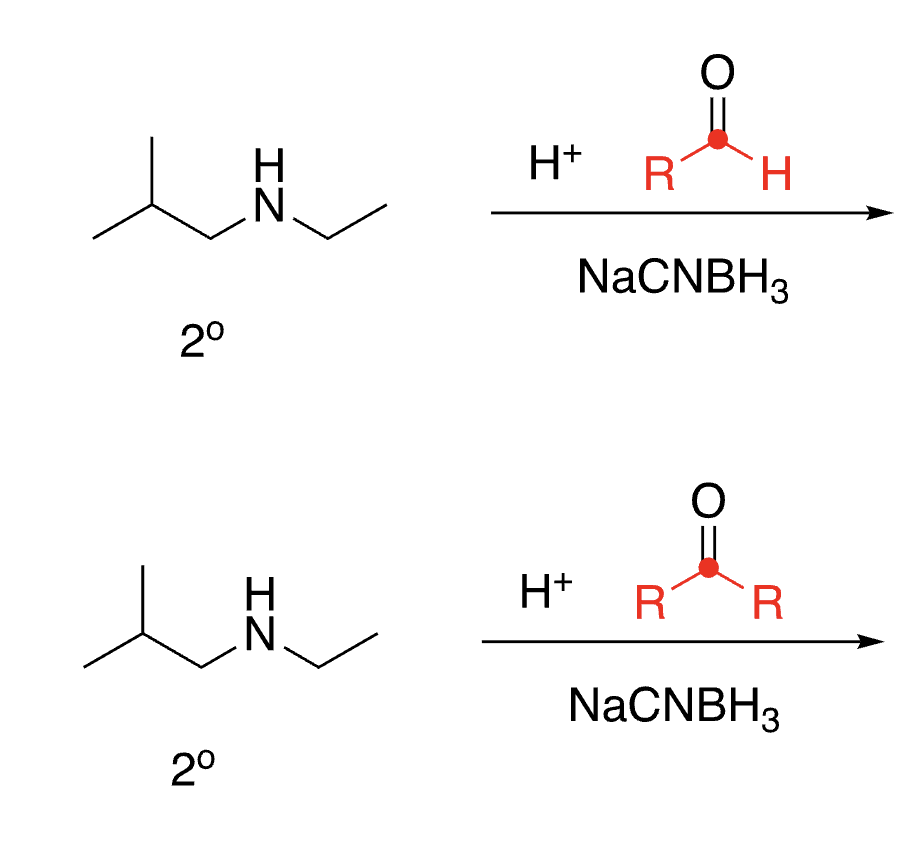
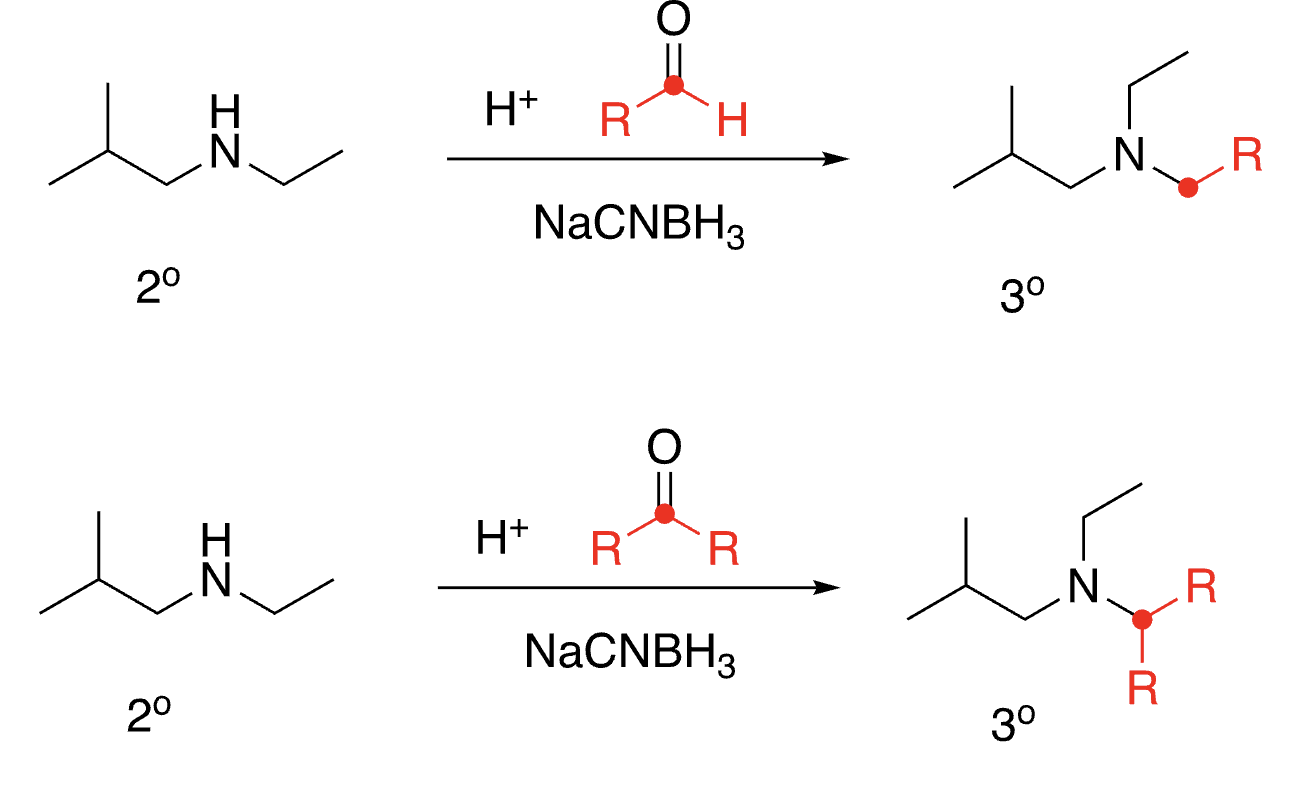
88
New cards
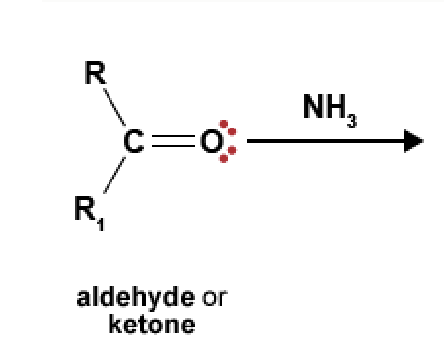
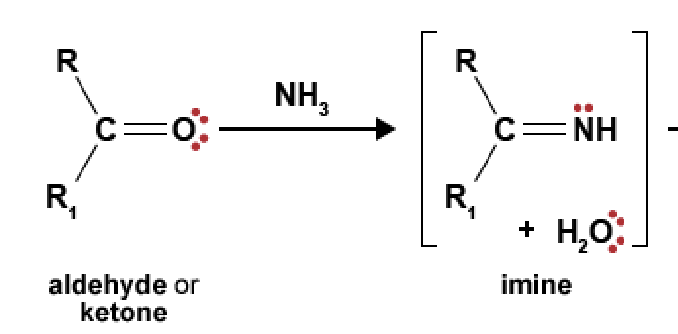
89
New cards
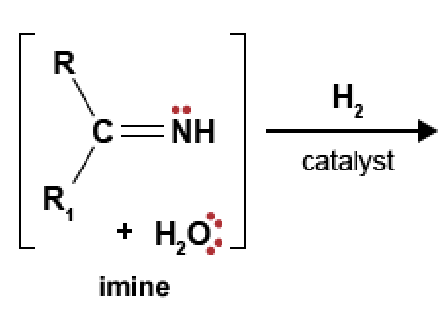
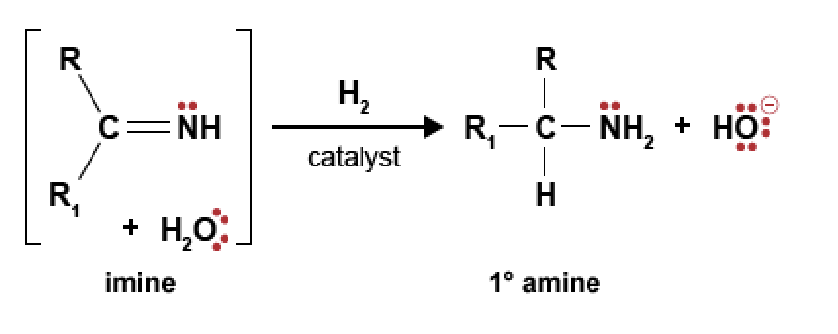
90
New cards
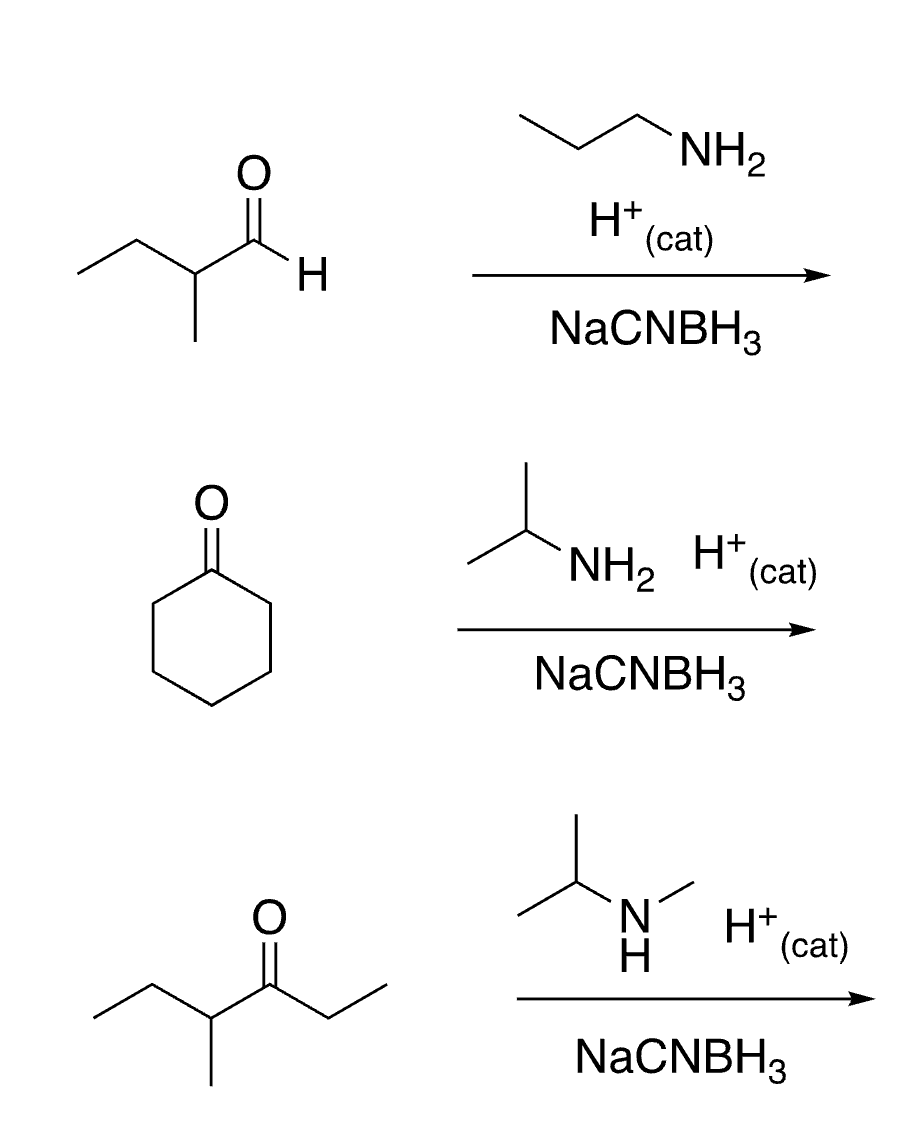
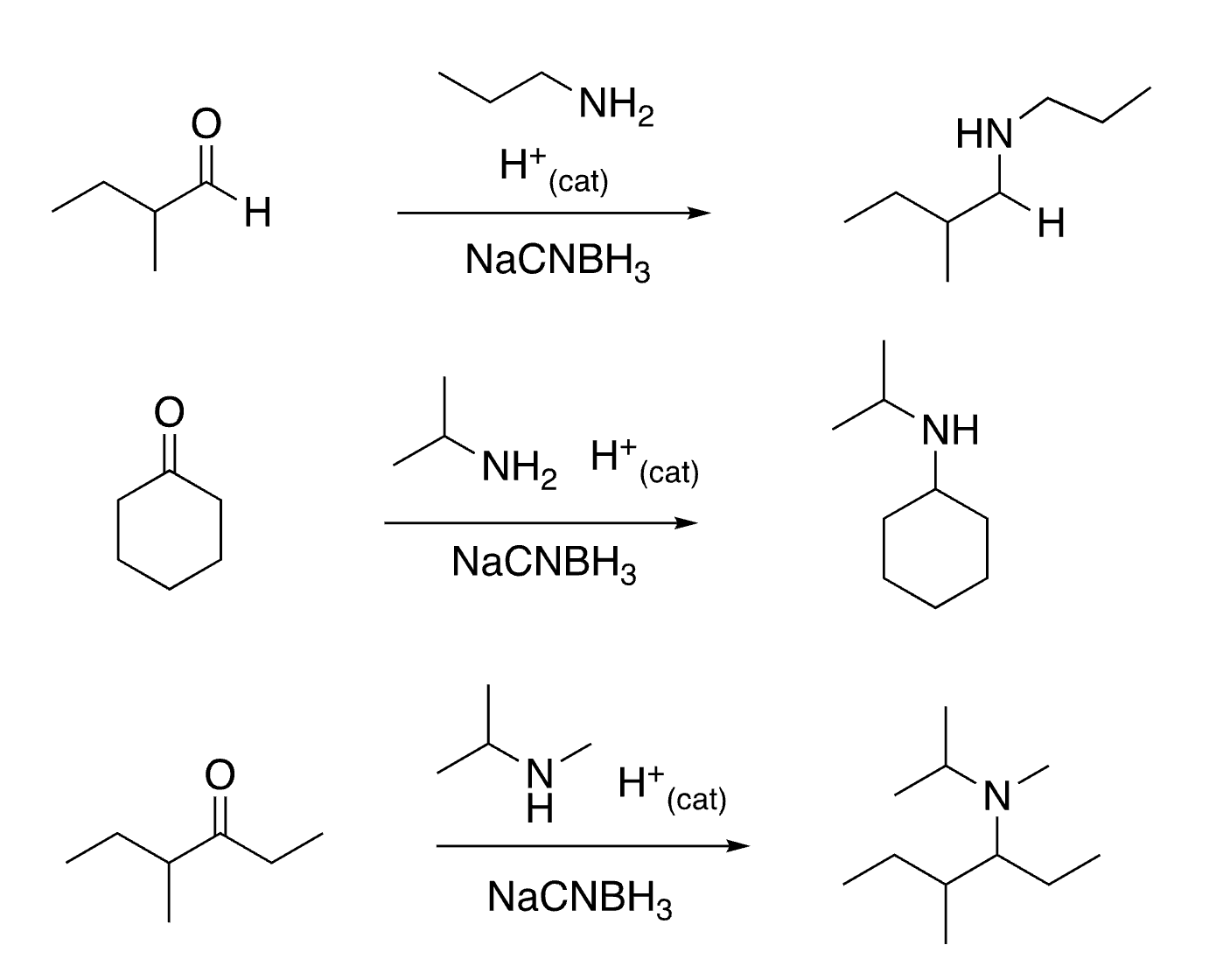
91
New cards
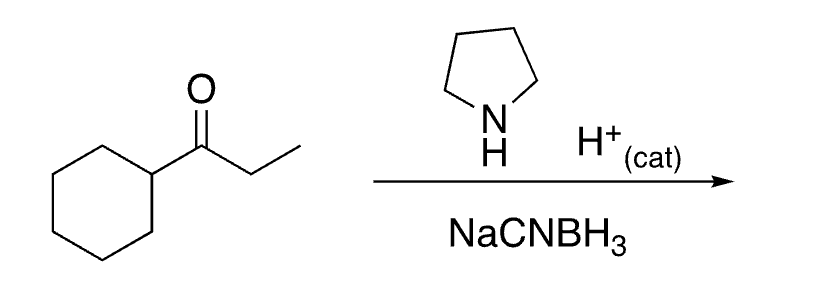
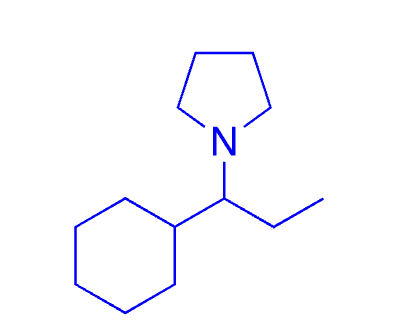
92
New cards
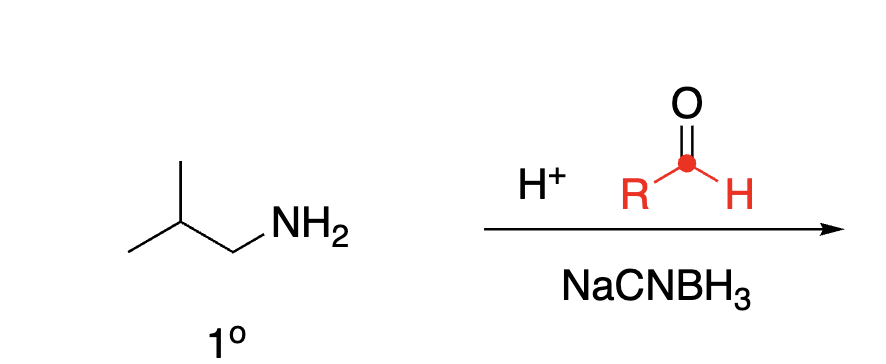
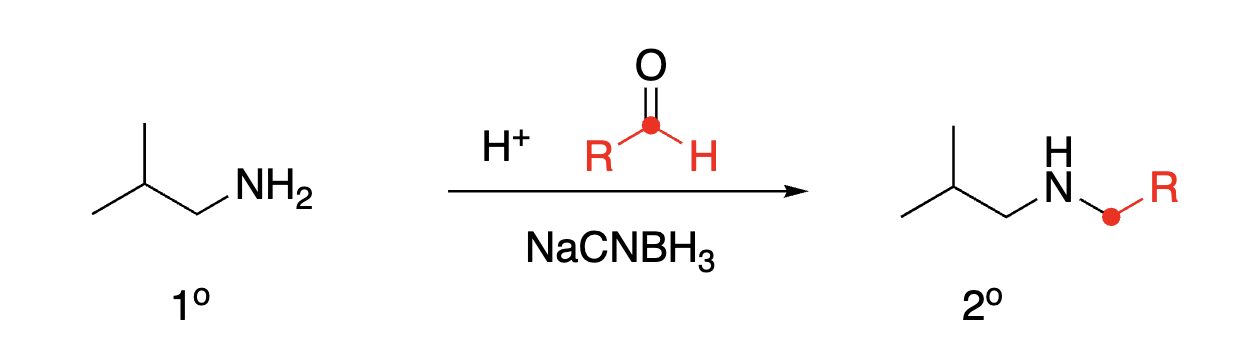
93
New cards
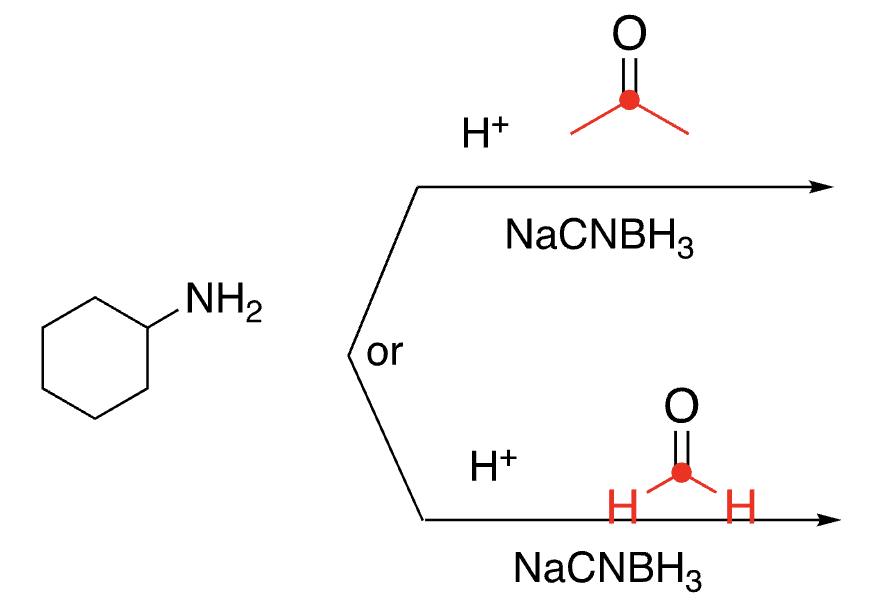
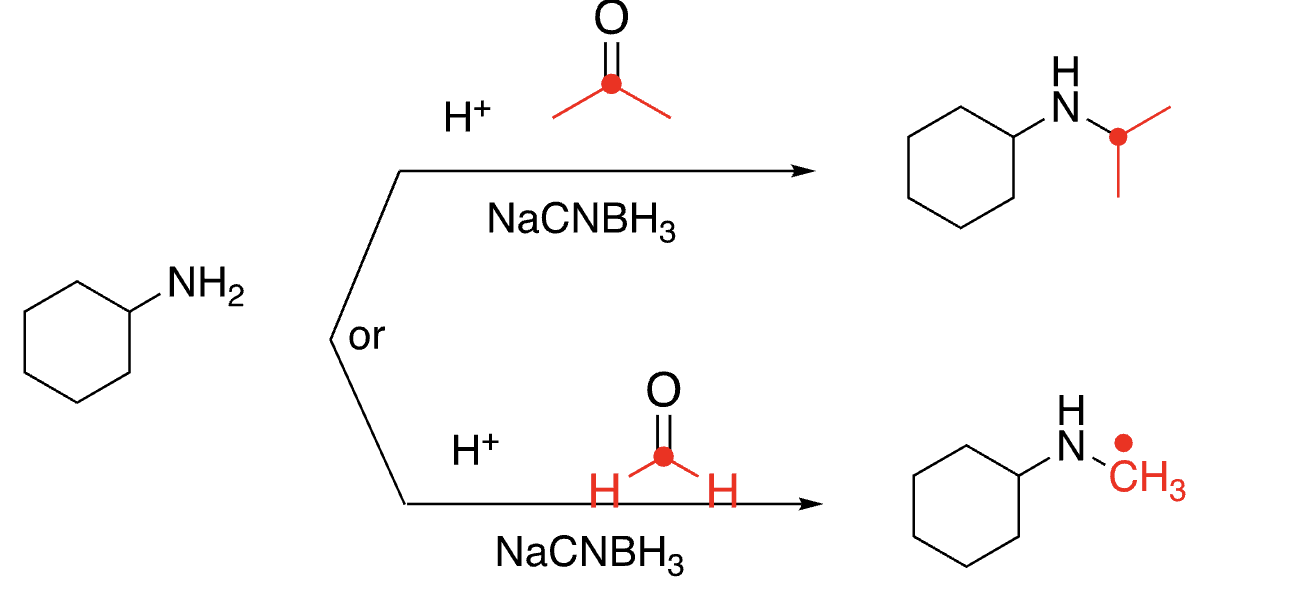
94
New cards
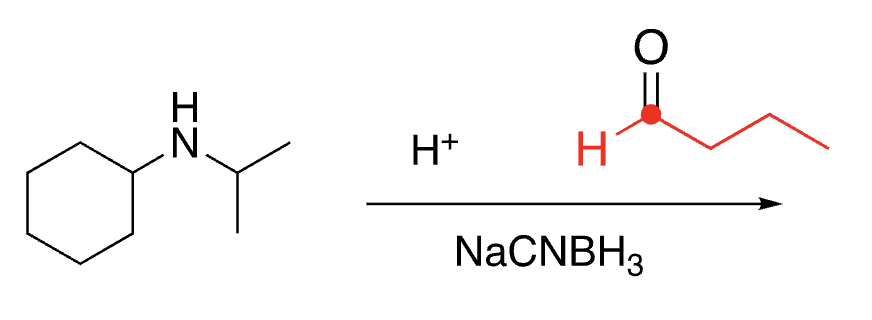
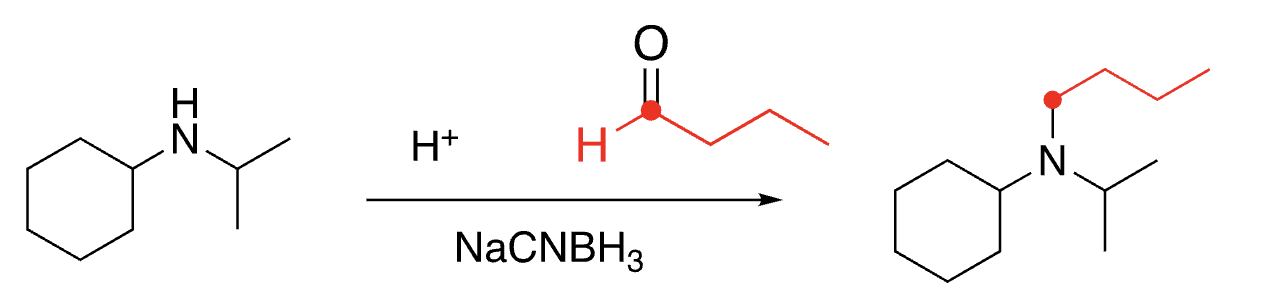
95
New cards
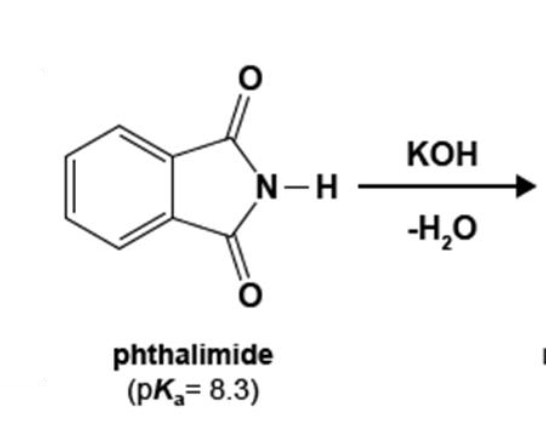
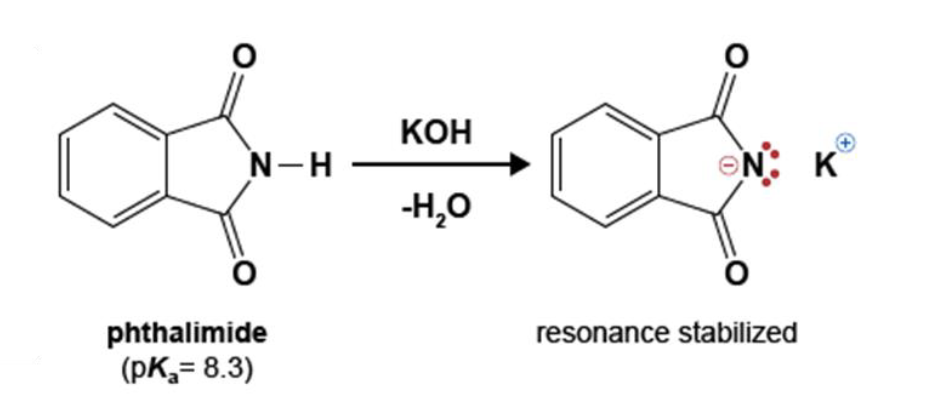
96
New cards
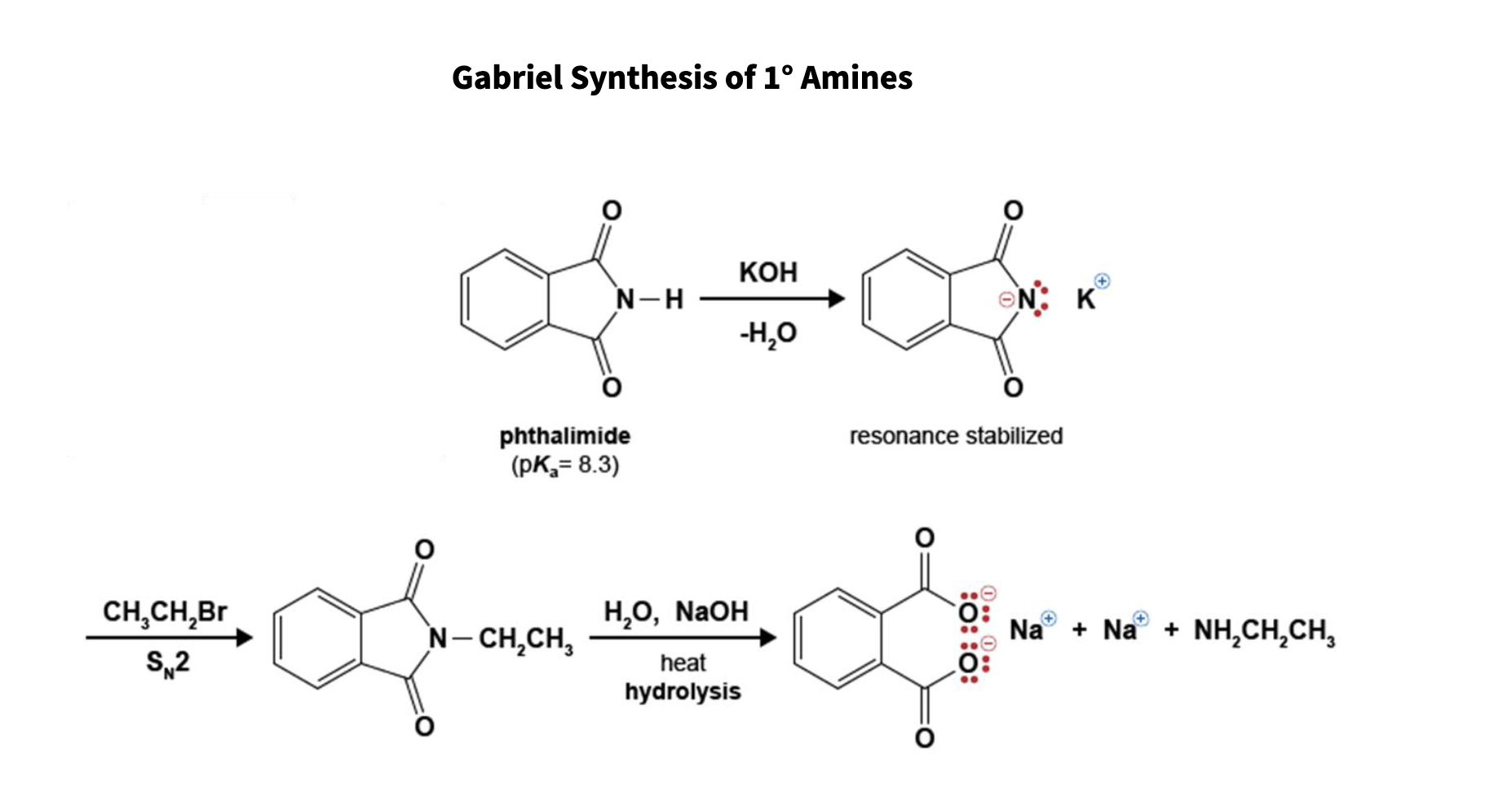
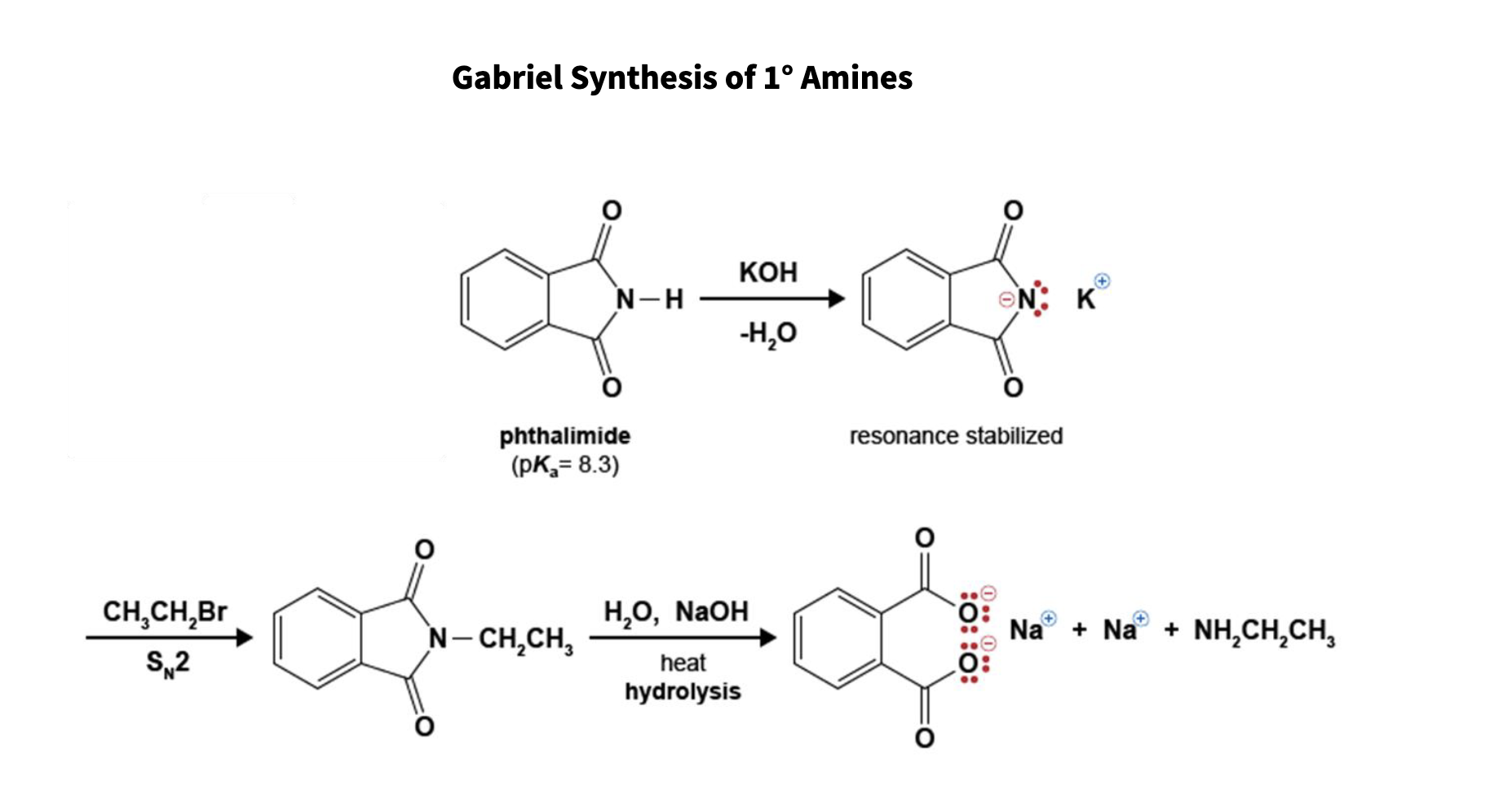
97
New cards