Lecture 4
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
What is a bronsted base
a proton acceptor
2
New cards
What is a lewis base ?
a reactant that acts as an electron air donor
3
New cards
when ammonia reacts with an acid what is formed
a salt
4
New cards
how are phenols different from alcohols?
the oH group is directly attached directly to an aromatic ring
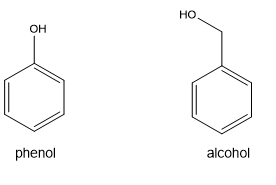
5
New cards
physical properties pf phenols
liquids
low melting salts
easily oxidised
6
New cards
why do meta and para nitrophenols have high boiling points
hydrogenbonding