Chapter 2 (Campbell's Biology in Focus)
Concept 2.1
- ==Matter== is anything that takes up space and has mass
- ==An element== is a substance that cannot be broken down into other substances by chemical reactions
- ==A compound== is a substance consisting of two or more different elements combined in a fixed ratio
- ==Essential elements== are natural elements that an organism needs to live a healthy life and reproduce
- ==Trace elements== are required by an organism in only minute quantities
Subatomic Particles
- The three main parts of an atom are
- neutrons
- protons
- electrons
- The unit of measurement for atoms and subatomic particles is a ==dalton==
- Different atomic forms of the same element are called ==isotopes==
- A ==radioactive isotope== is one in which the nucleus decays spontaneously giving particles and energy
- ==Potential energy== is the energy that matter possesses because of its location or structure
Electrons Distribution and Chemical Properties
- electrons are found in different electron shells
- The chemical behaviour of an atom depends mostly on the number of electrons in its outermost shell
- These electrons are called ==valence electrons== and are found in the ==valence shell==
The formation and function of molecules depend on chemical bonding between atoms
- Attractions between atoms are called ==chemical bonds==
- A ==covalent bond== is the sharing of a pair of valence electrons by two atoms
- Two or more atoms held together by covalent bond constitute a ==molecule==
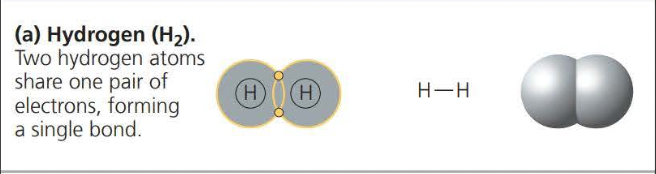
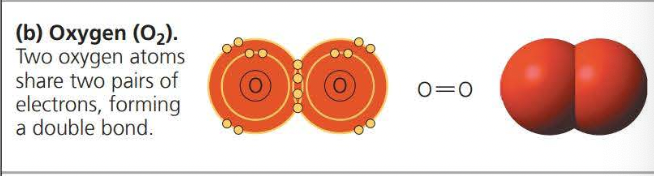
- The attraction of a particular atom for the electrons of a covalent bond is called its ==electronegativity==
- A ==nonpolar covalent bond== is when electrons are shared equally because two atoms have the same electronegativity
- A ==polar covalent bond== is when an atom is bonded to a more electronegative atom, the electrons will therefore not share equally
Ionic Bonds
- Two resulting oppositely charged are called ==ions==
- Positively charged results in a ==cation==
- Negatively charged results in an ==anion==
- A ==hydrogen bond== is formed when there is a noncovalent attraction between hydrogen and an electronegative atom
- ==Van Der Waals interactions== occur when atoms get just close enough that a few electrons in their valence shell (electron cloud) touch
Chemical reactions make and break chemical bonds
- The making and breaking of chemical bonds leading to changes in the composition of matter are called chemical reactions
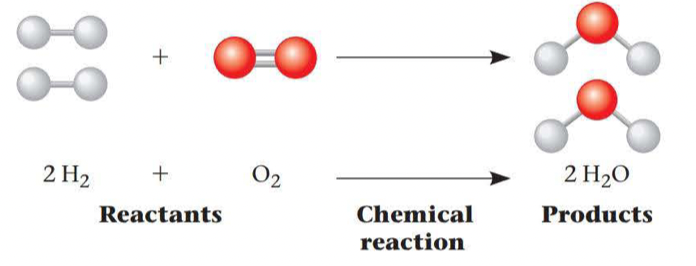
- The point at which the reactions offset one another exactly is called ==chemical equilibrium==
- The unequal sharing of electrons and water’s V-like shape make it a polar molecule.
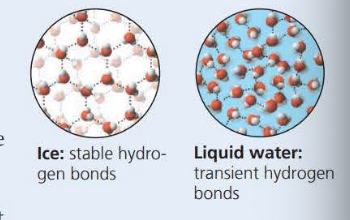
- The phenomenon of the ==cohesion== of water molecules is due to hydrogen bonds
- ==Adhesion== is the clinging of one substance to another
- ==Surface tension== is a measure of how difficult it is to stretch or break the surface of a liquid
- Anything that moves has ==kinetic energy==
- The ==specific heat== of a substance is defined as the amount of heat that must be absorbed or lost for 1g of that substance to change its temperature by 1C
- ==The heat of vaporization== is the quantity of heat a liquid must absorb for 1g of it to be converted from the liquid to the gaseous state
- ==Evaporative cooling== occurs because the hottest molecules, (those with the greatest kinetic energy) are the ones most likely to leave as a gas
Water: The Solvent of Life
- ==A solution== is a liquid that is a completely homogeneous mixture of two or more substances
- ==A solvent== is the dissolving agent of a solution
- ==The solute== is the dissolving agent of a solution
- ==An aqueous solution== is one in which the solute is dissolved in water, water is the solvent
- A ==hydration shell== is formed when there’s a sphere of water molecules around each dissolved ion
- Any substance that has an affinity for water is said to be ==hydrophilic==
- Substances that are nonionic and nonpolar are ==hydrophobic==
- ==Molarity== is the number of moles of solute per liter of solution
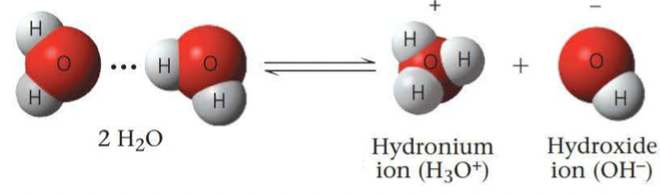
- ==An acid== is a substance that increases the hydrogen ion concentration of a solution
- A substance that reduces the hydrogen ion concentration of a solution is called ==a base==
Properties of water:
Anomalous expansion of water
High specific heat capacity (evaporative cooling and heat of vaporization)
Hydrogen bonds (pattern when it freezes)
Its versatility of it as a solvent because of the polar molecules
Cohesion (surface tension)