26.1 - discovery of nucleus
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
what was the accepted atomic theory before rutherford’s?
thomson’s plum pudding model - that the atom was composed of negatively charged electrons floating in a ‘sea’ of positive matter with no concentration of mass
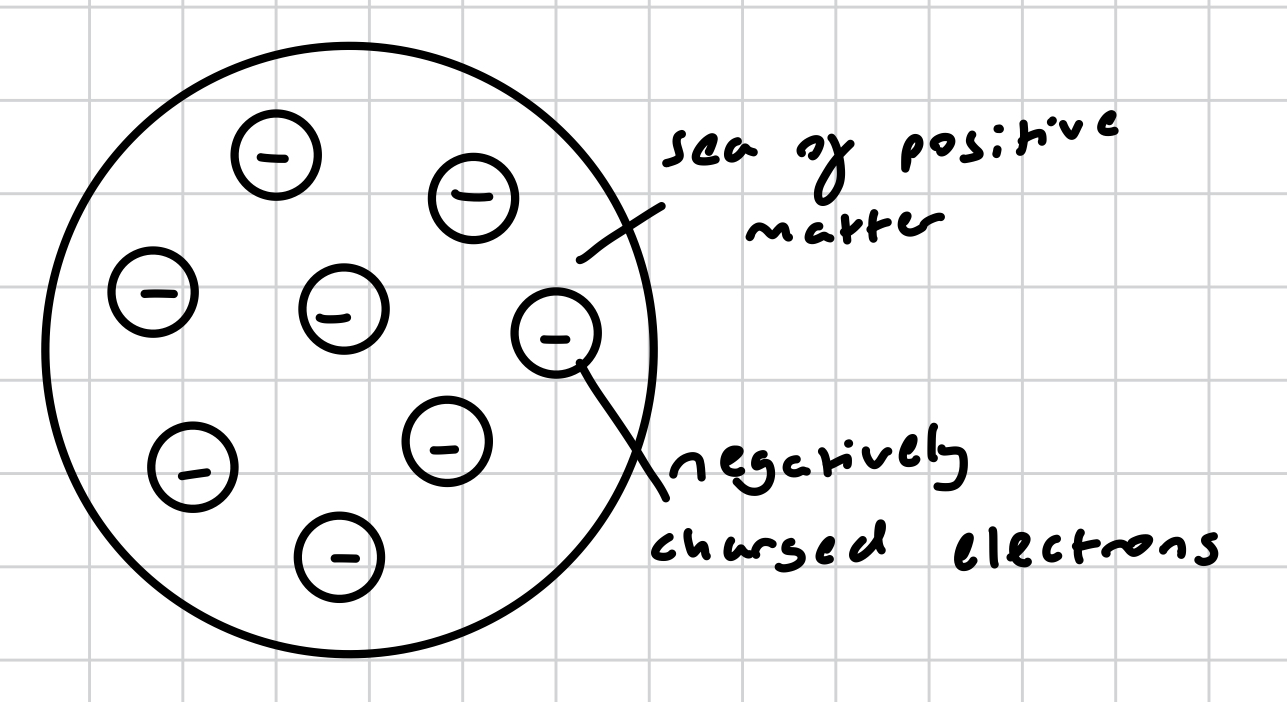
what was the order of discovery for subatomic particles?
electron
proton
neutron
what is rutherford’s atomic model?
mass concentrated in the center, in the nucleus
nucleus composed of protons and neutrons
positive charge concentrated in the center, in the nucleus
negatively charged electrons orbiting the nucleus in shells
strong nuclear force holding the nucleus together, overcoming the electrostatic repulsion of the protons
atom is majority empty space
nucleus is roughly 1/10,000 of the size of the atom
thomson vs rutherford
PICTURE TABLE
thomson vs rutherford similarities vs differences
PICTURE TABLE
what did rutherford know about radiation?
of it’s existence
why particles radiated
the 3 kinds of radiation
what was the set up for the gold leaf / rutherford’s scattering experiment?
PICTURE
MUST INCLUDE
wall / detectors goes all around the apparatus, at a constant distance from the point of impact between beam and metal foil
alpha particles come from an alpha source
the metal container is evacuated
it is a ‘thin gold sheet / foil / leaf’
what happened in rutherford’s scattering experiment?
alpha particles, of the same Ek, fired at a thin gold leaf foil
majority went straight through
1/2000 particles deflected, 1/10,000 deflected at an angle >90°
number of alpha particles reaching the detectors per minute for different angles of reflection are record

⠀
⠀
what did rutherford’s scattering experiment prove?
the atom was made up of mostly empty space, and that mass was concentrated in the centre, as the majority of alpha particles fired straight through without deflection
that the nucleus was roughly 1/10,000 of the size of the atom as 1/10,000 alpha particles would deflect at an angle >90°
the nucleus was positively charged because the alpha particles that were deflected at an angle >90° must’ve gone under electrostatic repulsion to be deflected in such a way
the existence of the nucleus, for the reasons previously mentioned
that the existing atomic theory (thomson’s plum pudding) could be disproved
which particle was used in the gold leaf experiment?
alpha particles (helium nucleus)
in rutherford’s scattering experiment, why was gold used?
it was the only material that could be rolled thin without cracking
in rutherford’s scattering experiment, why did the gold foil have to be thin?
the reduce the layers of atoms as much as possible as that would increase the chance of an alpha particle hitting the nucleus, since the chances of that are 1/10,000n (n = number of layers of atoms)
what are some features of the alpha particles used in rutherford’s scattering experiment?
alpha source had a long half-life
all alpha particles were fired with the same kinetic energy
they were shot in a narrow beam
in rutherford’s scattering experiment, why was it important that all alpha particles were shot with the same Ek?
because slow particle would deflect more than fast particles and that would interfere with the results
in rutherford’s scattering experiment, what does it mean for the metal container to be ‘evacuated’ and why was this important?
evacuated means there was no air inside
to prevent air absorbing the alpha particles before they reached the gold foil; prevent deflection ionisation
deflection due to electrostatic forces diagram;
IMAGE

⠀
⠀
in rutherford’s scattering experiment, why is it important that the wall / detectors can rotate completely around the apparatus?
so it can detect the deflected alpha particles in whatever direction they are scattered
in rutherford’s scattering experiment, why was it important that the alpha source had a long half-life?
otherwise later readings would be lower than earlier readings due to radioactive decay of the source nuclei
in rutherford’s scattering experiment, why was it important that alpha particles were fired in a narrow beam?
to increase precision and reduce the likelihood of multiple scattering events - which would make it harder to observe the scattering (i.e., the precise angle of deflection) of an individual alpha particle, which was needed to deduce the structure of the atom

⠀
⠀⠀
how many alpha particles are deflected?
1/2000
how many alpha particles are deflected at angle >90°?
1/10,000
what causes 180° deflection?
‘head-on’ collision of the alpha particle with the nucleus

⠀
⠀
what is the diameter of a nucleus measured in?
femtometres (fm), x10¹⁵ m
⠀
⠀
what causes greater deflection?
increased electrostatic repulsion of the alpha particle and the neutron
decreased separation (i.e., closer proximity) of the alpha particle and the nucleus
smaller least distance of approach (closer they are) = greater the electrostatic force of repulsion = more deflection
what is the least distance of approach?
the closest an alpha particle can get to a positively charged nucleus before the electrostatic force of repulsion deflects the alpha particle back
what happens the closer the alpha particle is to ‘head-on’ collision?
the greater the deflection - since electrostatic repulsion (therefore deflection) increases with decreased separation
the smaller the least distance of approach
DIAGRAM
what is the magnitude of charge of a nucleus?
+Ze
+ = nucleus has a positive charge
Z = atomic number of element (number of protons)
e = electron charge (protons and electrons have the same charge)
where does the equation for magnitude of charge of an electron come from?
using coulomb’s law of force and neutron’s laws of motion
FURTHER RESEARCH NEEDED
what is the symbol for ‘number of layers of atoms’?
n
what is the probability that an alpha particle hits the nucleus?
1/10,000n
n is the layers of atoms
DIAGRAM
what does the probability that an alpha particle hits the nucleus depend on?
the effective area of the cross-section of the nucleus as compared to that atom
CLARIFY
what is the area ratio for a nucleus of diameter (d) in an atom of diameter (D)?
¼πd² / ¼πD² (area of nucleus / area of atom),
rearranged into d² = D² / 10,000n
LOOK INTO DERIVATION
when is the alpha particle at the least distance of approach to the nucleus?
in a head-on collision
what is the kinetic energy of an alpha particle in head-on collisions?
in the least distance of approach, the Ek of the alpha = the Ep of the alpha in the nucleus’ electric field
Ek = (QαQ𝑛) / (4πε0d)
Qα = alpha charge
Q𝑛 = nucleus charge (+Ze)
ε0 = vacuum permittivity of free space, 8.85 × 10–12 F m–1
d = distance of approach
what is (vacuum) permittivity of free space (ε0)?
a measure of how easy it is to generate an electric field in a certain material
a constant
8.85 × 10–12 F m–1
