ht quiz 8
1/23
Earn XP
Description and Tags
boiling heat transfer
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is Boiling Heat Transfer?
occurs when temp of surface is sufficiently higher than TSAT of liquid its in contact with
transforms some of it into vapour
most of energy which is transferred from surface to liquid is absorbed by change in phase from liquid to vapour
what is hfg
latent heat of vaporisation
Why is boiling heat transfer such an effective method of removing thermal energy from a surface?
in single phase ht, thermal energy absorbed by fluid from hot surface increases temp of fluid
more gentle temp gradient at surface
reduces rate of wall ht
in boiling ht, due to high hfg needed to convert a unit mass of liquid into vapour
most of E absorbed by fluid from hot surface used to convert liquid to vapour
maintains steep temp gradient at surface
high wall heat flux rates
How does the pressure inside a vapour bubble relate to the surrounding pressure?
pressure inside > surroundings
difference between pressures inside and outside bubble is:
proportional to surface tension of liquid
inversely proportional to radius of bubble
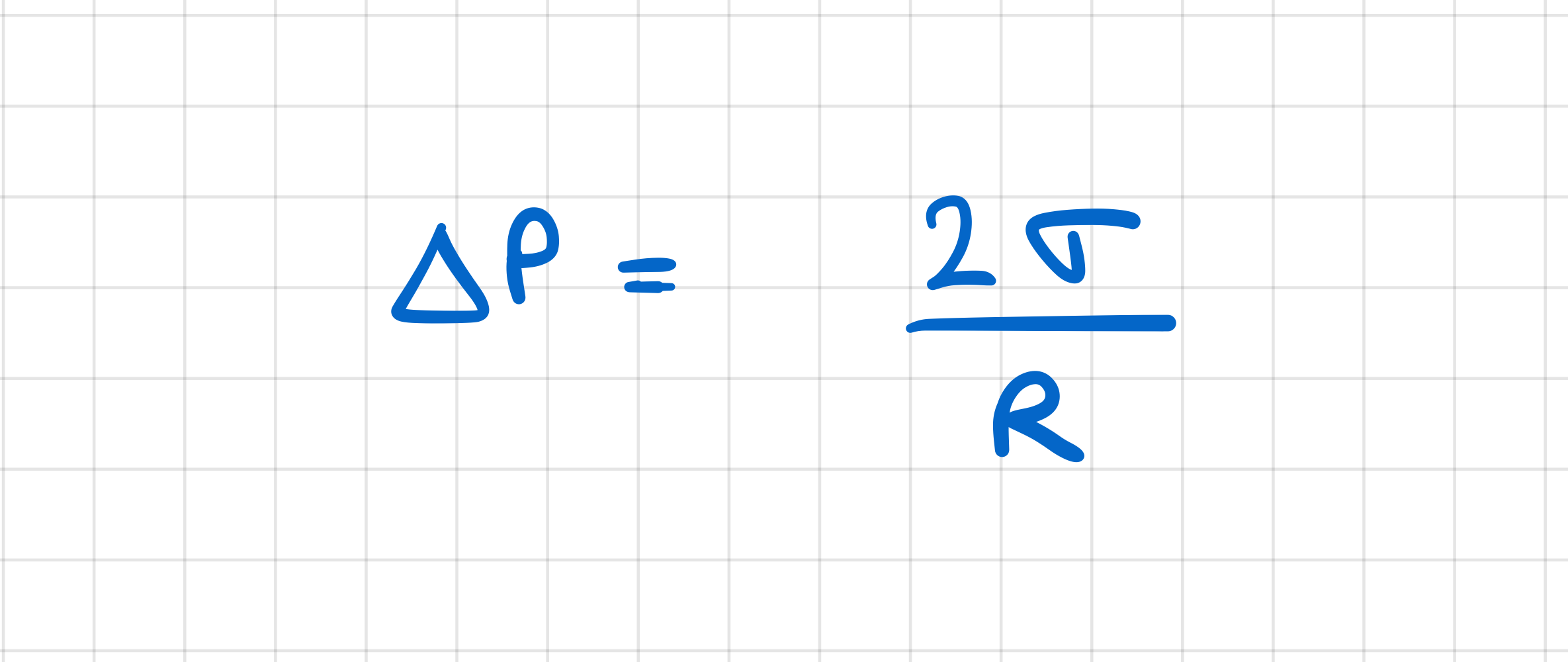
What condition needs to be satisfied for a vapour bubble to grow?
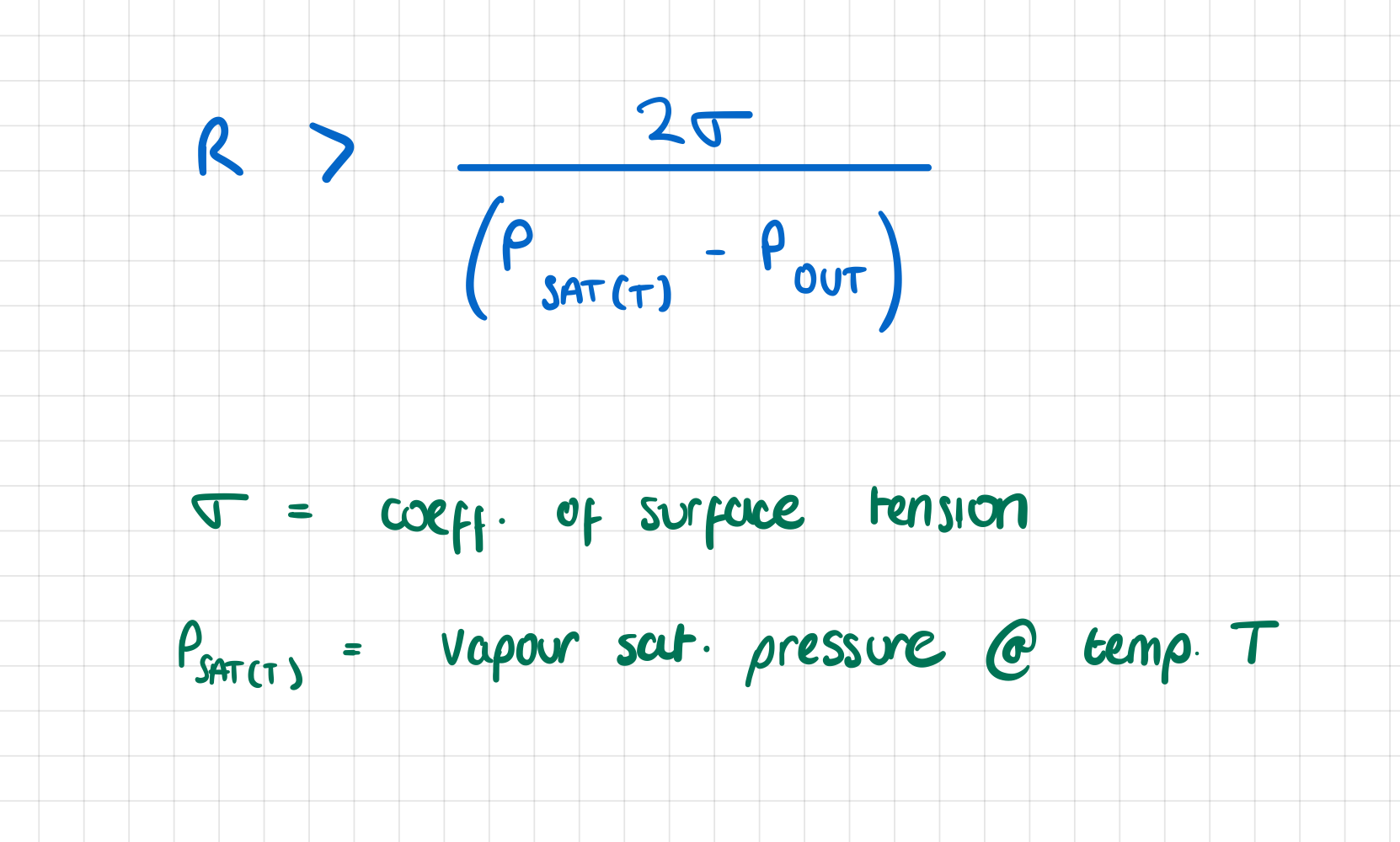
Why is it necessary for the wall temperature to be several degrees higher than the saturation temperature in order to have boiling?
if wall temp = vapour TSAT , PSAT = surroundings
only bubbles with infinitely large radius would survive
bc increase in P across bubble surface is
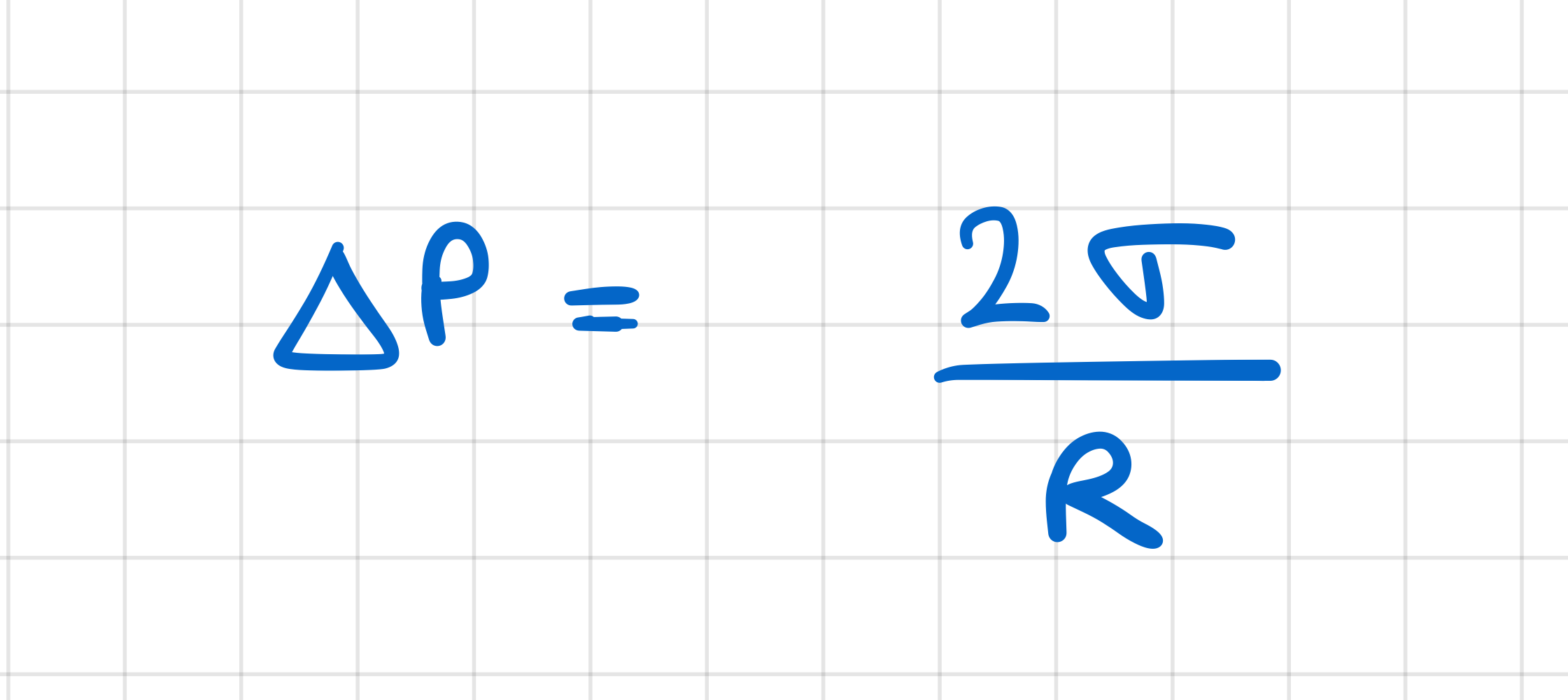
for boiling to start, surface temp needs to be high enough for critical radius to be same sixe as surface cavities (which act as nucleation sites)
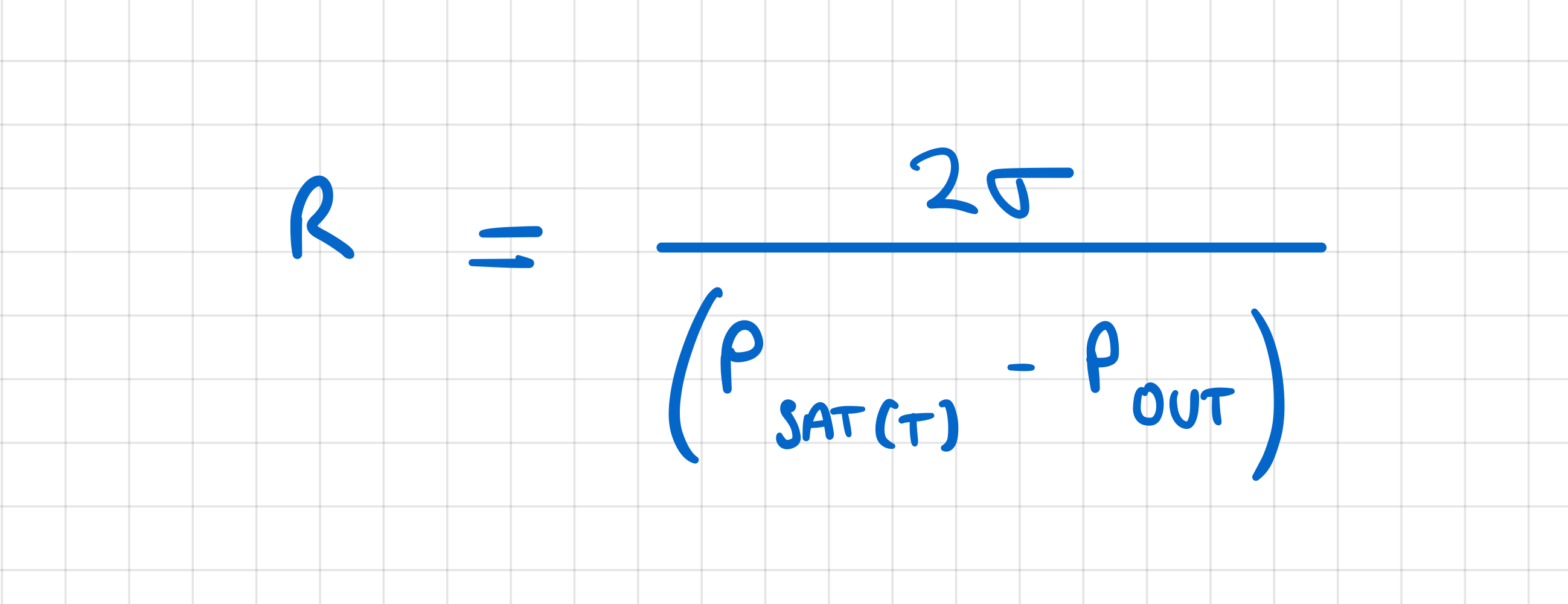
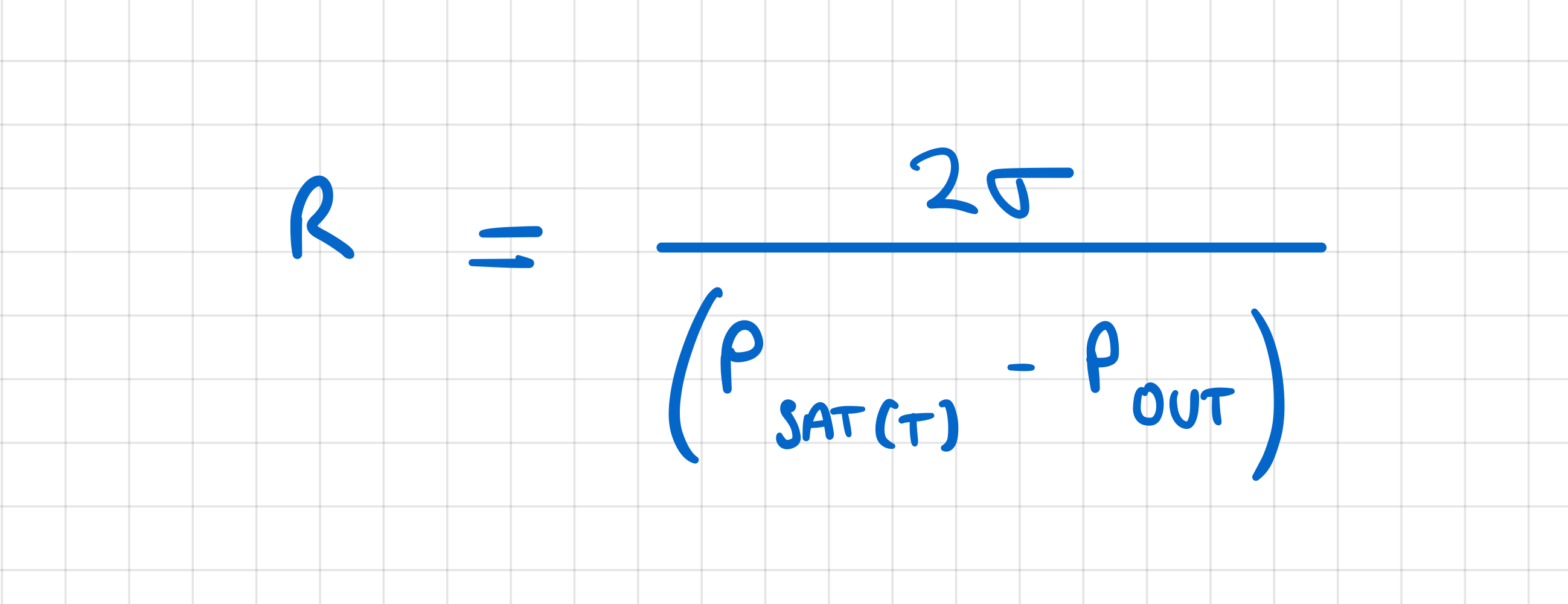
Which are the dimensionless groups which characterise boiling heat transfer?
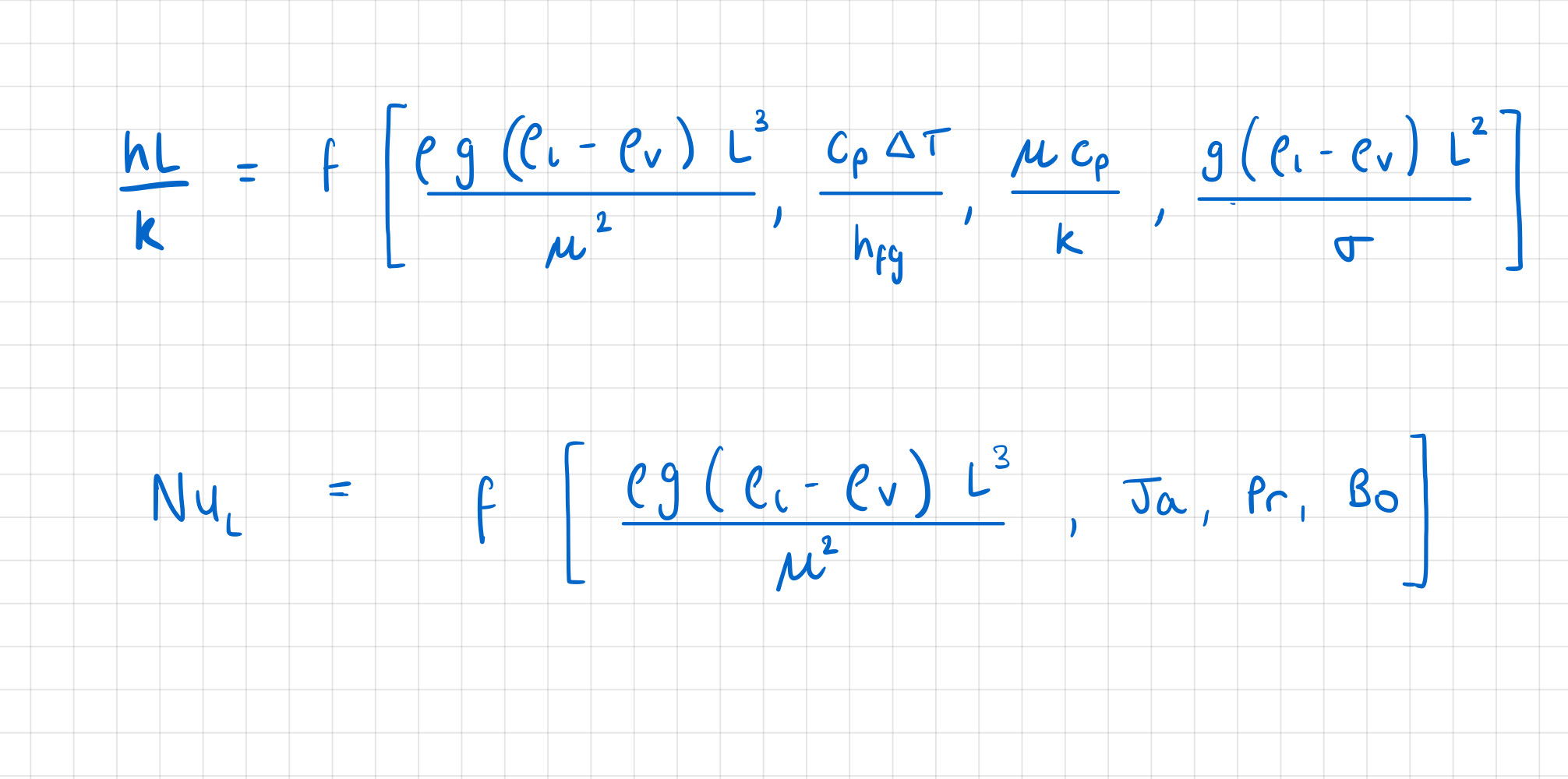
Explain the term Pool Boiling
initially stagnant pool of liquid is heated to its boiling point
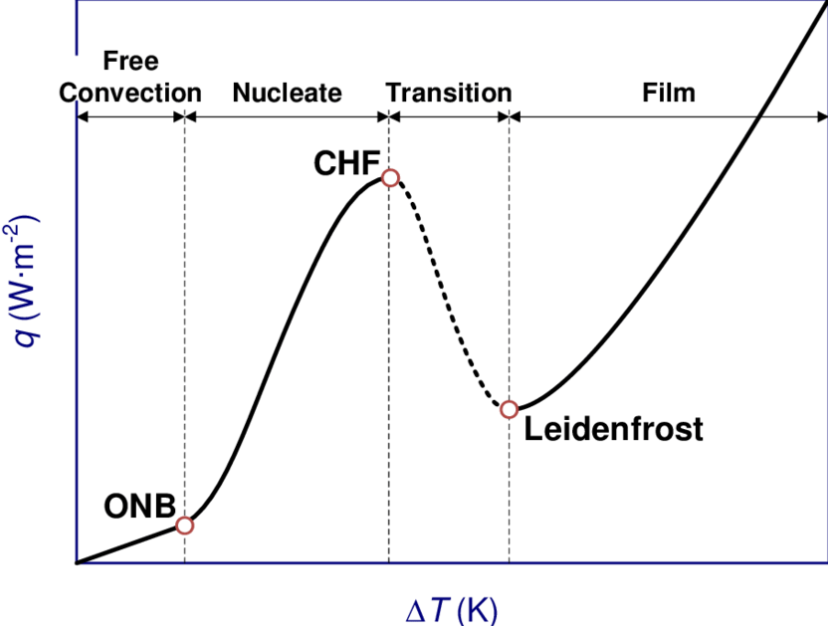
List the four main regimes encountered in pool boiling, as the difference between the temperature of the heating surface and the liquid saturation temperature is gradually increased.
natural convection
nucleate boiling
transition boiling
film boiling
How can you explain the strong noise generated in kettles during the boiling process?
when temp of liquid above heated surface < boiling temp
vapour bubbles generated at heated surface collapse (re-condense)
as they rise through sub-cooled liquid
the collapsing is the noise
In pool boiling, controlled through the gradual increase of the difference between the temperature of the heating surface and the liquid saturation temperature, identify the regime in which an increase in the difference between the difference in surface and liquid saturation temperature leads to a reduction in surface heat flux rate and explain why.
transition boiling regime
increase in surface and liquid TSAT = reduction in wall heat flux rate
because
in this region, large proportion of heated surface covered by vapour film
vapour film acts as thermal barrier
as surface temp increases, proportion of surface covered by film increases
reduces wall heat flux rate
at min. heat fux point, entire surface covered by vapour film
beyond this point, film boiling regime takes over
wall heat flux rate increases with surface temp
In pool boiling, controlled through the gradual increase of the wall heat flux rate, explain what the Burnout Point is.
as heat flux rate increases
TW - TSAT also increases at first
through natural convection and nucleate boiling regimes
at end of nucleate boiling regime, further increase in wall heat flux will need a large increase in wall temp to sustain it
causes sudden change from nucleate boiling to film boiling regime
the sudden jump in surface temp raises temp above melting point of surface material
so, at end of nucleate boiling regime, the point where qw reaches its local max = burn out point
For the Film Boiling regime, what are the modes of heat transfer from the heated wall to the liquid?
from heated surface to boiling liquid, across vapour film
via. heat conduction
as temp diff. increases, thermal radiation also contributes
In empirical heat transfer correlations for the Film Boiling regime, how is the augmented latent heat of vapourisation and why is there a need for it.
augmented latent heat of vaporisation = h’fg
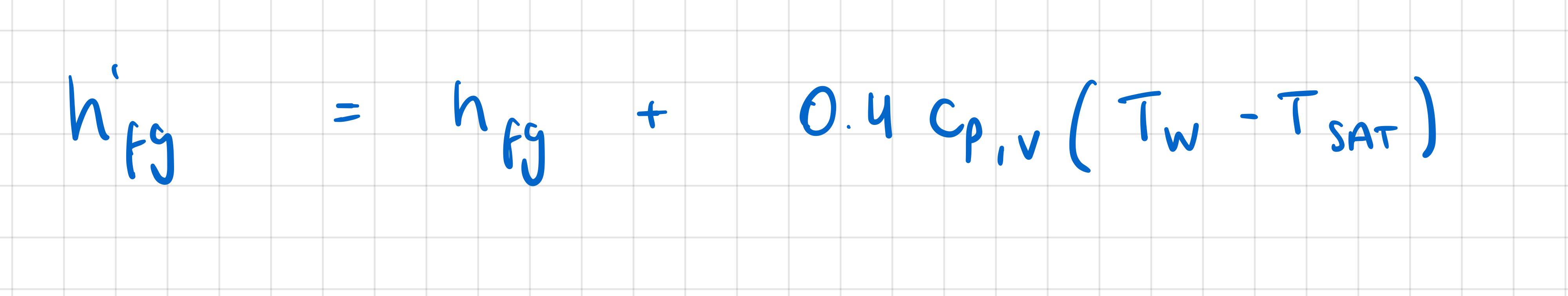
accounts for energy needed to change liquid → vapour
AND
for energy needed to raise its temp across film
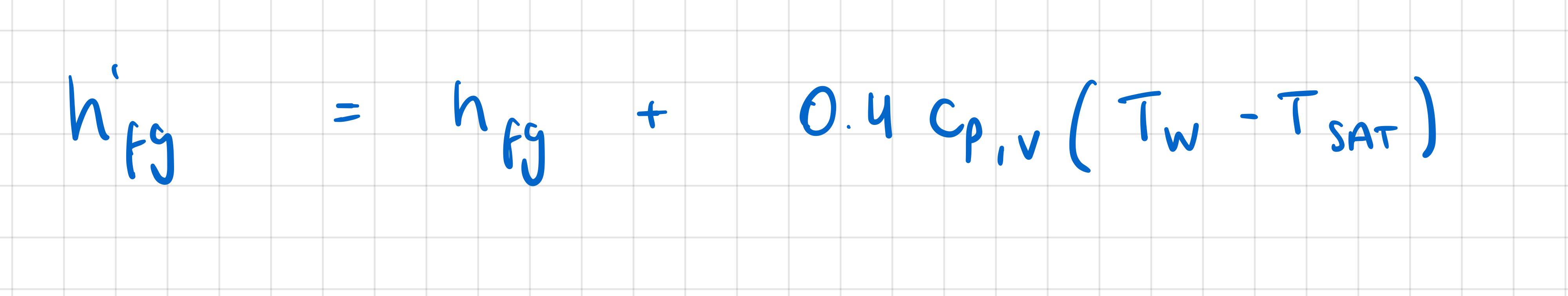
In empirical heat transfer correlations for the Film Boiling regime, at what temperature are the vapour properties defined and why?
vapour properties defined at film temp = av of wall and saturation temps, because there is large diff between the two
[not necessary for nucleate boiling regime bc diff between wall and saturation temps is small]
Explain convection boiling
convection boiling occurs when liquid forced to flow past heated surface
which is at temp > liquid TSAT
general effects of imposed fluid motion on boiling ht
imposed fluid motion increases coefficient of wall heat flux
increases qmax (the critical wall heat flux)
In external convection boiling over a flat surface:
how is the wall heat flux q calculated in the nucleate boiling regime
wall heat flux q in nucleate boiling regime
caculated through superposition method
add qb (nucleate boiling ht for pool boiling) to qc (forced convection ht
q = qb + qc
which two dimensionless groups determine augmentation of critical heat flux qmax in external convection boiling?
Webber Number
ratio between liquid and vapour densities
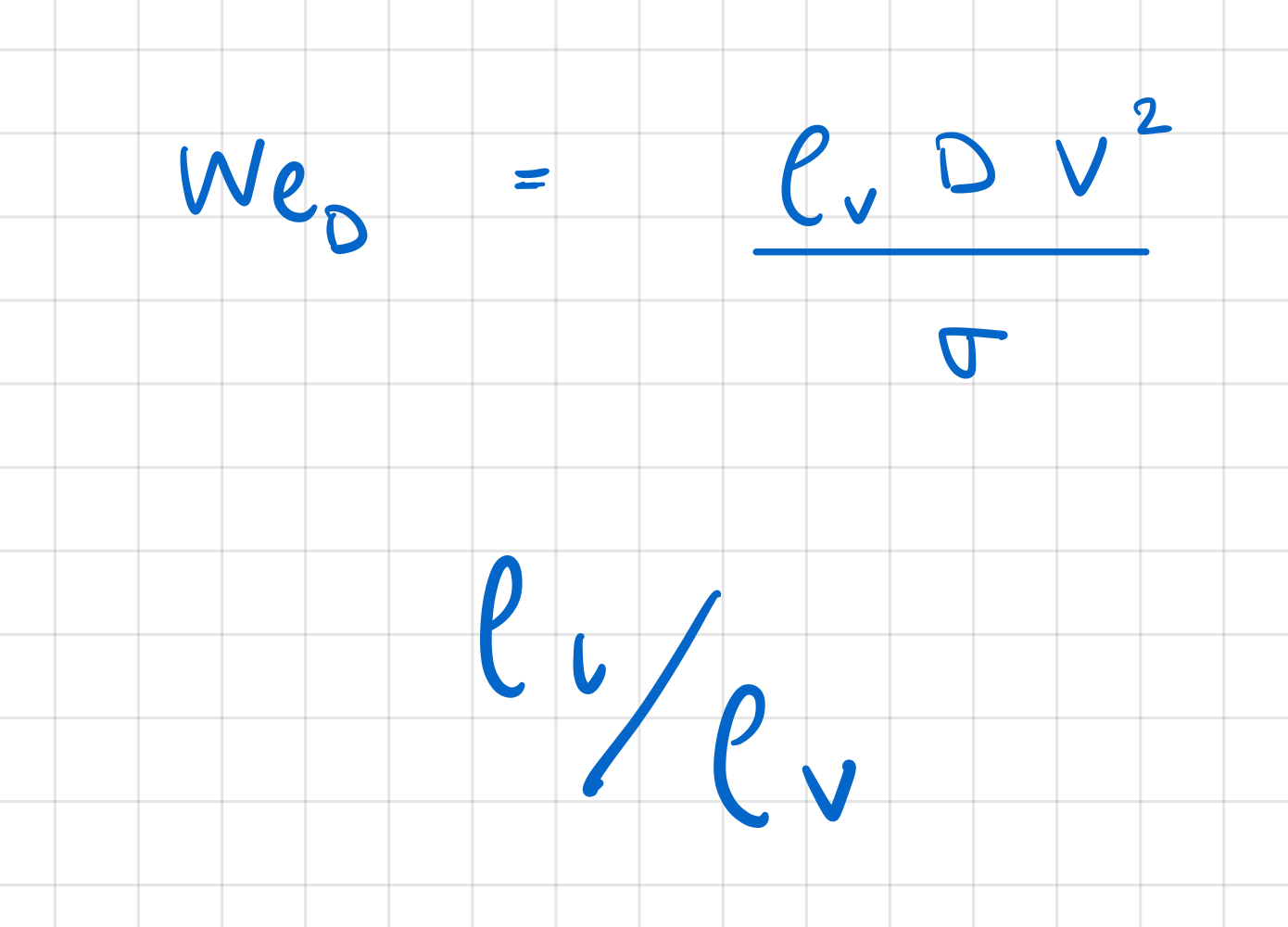
In internal forced convection boiling in an upward flow within a heated tube, with sub- cooled liquid at the inlet, list the flow regimes that develop, provided the tube is long enough.
single phase flow
bubbly flow
slug flow
annular flow
annular flow with entrainment
drop flow
single phase vapour
In internal forced convection boiling in an upward flow within a heated tube, with sub- cooled liquid at the inlet, list the modes of heat transfer that develop, provided the tube is long enough.
heat convection to liquid
sub-cooled boiling
saturated nucleate boiling
forced convective ht through annular liquid film
forced convective transfer to liquid deficient region
heat convection to vapour
In internal forced convection boiling in an upward flow within a heated tube, what is the Dry Out point and what is its practical significance under uniform heating conditions?
Dry out point = end of annular liquid film that covers heated surface of tube
practical significance = locartion of abrupt increase in wall temp
In internal forced convection boiling in an upward flow within a heated vertical tube which dimensionless groups are used in the calculation of the ratio of the actual local heat flux coefficient to that of fully-developed forced convection, based on the liquid properties?
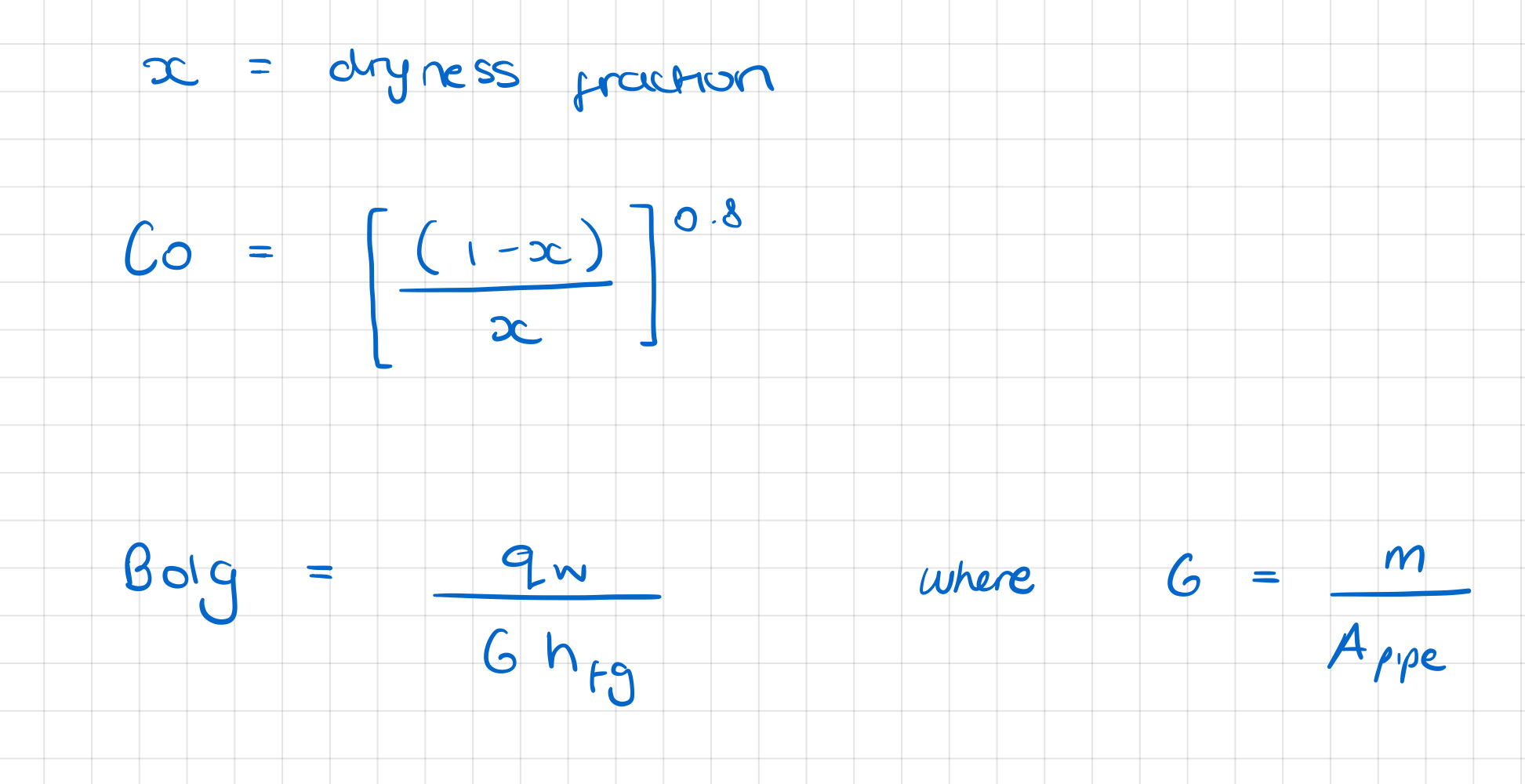
In internal forced convection boiling in flow within a heated horizontal tube, how is the ratio of the actual local heat flux coefficient to that of fully-developed forced convection, based on the liquid properties calculated?
dependa on Froude number:
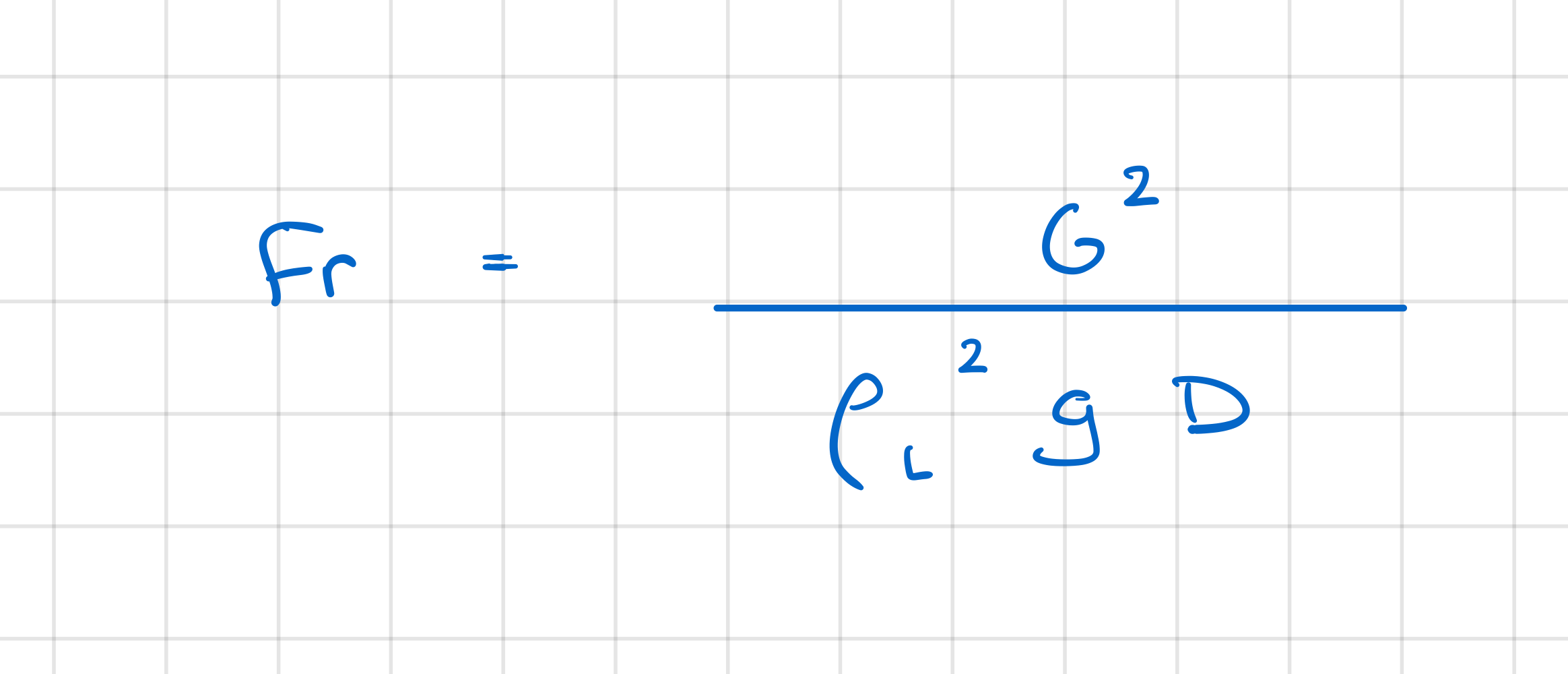
Fr > 0.04, high speeds
gravitational effect negligible
flow remains symmetric
calculation procedure identical to vertical tube calc
Fr < 0.04, low speeds
gravitational effects lead to non-symmetric flow
Fr number included in correlation for heat flux ratio