Section 16 - Anemias Caused by Impaired Production of RBCs
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Anemia
A condition in which there is reduced oxygen delivery to the tissues
May result from:
Increased RBC loss- destruction
Decreased Production of RBC
Look for:
Schistocytes,
Icteric blood (bilirubin)
Changes in MCHC
Hgb in urine
Increased MCV
Classification of Anemia
1) Morphologically- MCV or MCHC, helpful when looking at CBC results on case studies
2) Pathophysiologically- what is happening to the production of RBC/Hgb
Iron Notes
2/3 of total body Iron is found in Hgb
Our Fe is repeatedly recycled. Very small amount lost. It’s replaced by diet
Men and women have very different Fe requirements
Kids require more due to increased growth rates
Blood loss necessitates greater Fe need
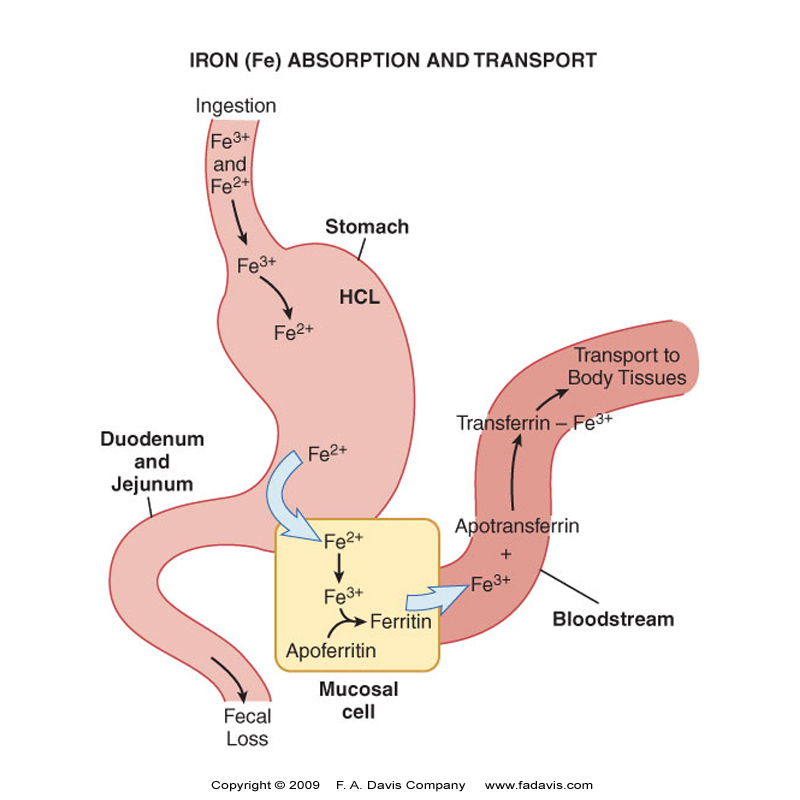
Iron metabolism
Bioavailability of ingested Fe is 5-17%
Most dietary Fe is in the Ferric (+3) state → ferrous state (2+) be reductase enzymes like Duodenal Cytochrome B (DCYTB) for optimal absorption
Fe is carried into the enterocytes of the intestinal lining by Divalent Metal Transporter 1 (DMT1)
In enterocyte Fe 2+ oxidized to Fe 3+ by Hephaestin
Enterocytes can either store Fe as Ferritin (apoferritin + Fe 3+) or move into the rest of the body
As required by the body, Fe is exported by Ferroportin 1 FPN1 across enterocyte membrane
Ferroportin 1 (FPN1)
Protein that transports Fe across cell membranes
Carries Iron from Enterocytes, macrophages, and hepatocytes into blood stream
Regulated by Hepcidin
***Takes Fe out of enterocytes and puts it into the BLOOD STREAM. Also takes Fe out of macrophages and liver
Removes Fe from
1) Enterocytes
2) Macrophages
3) The liver
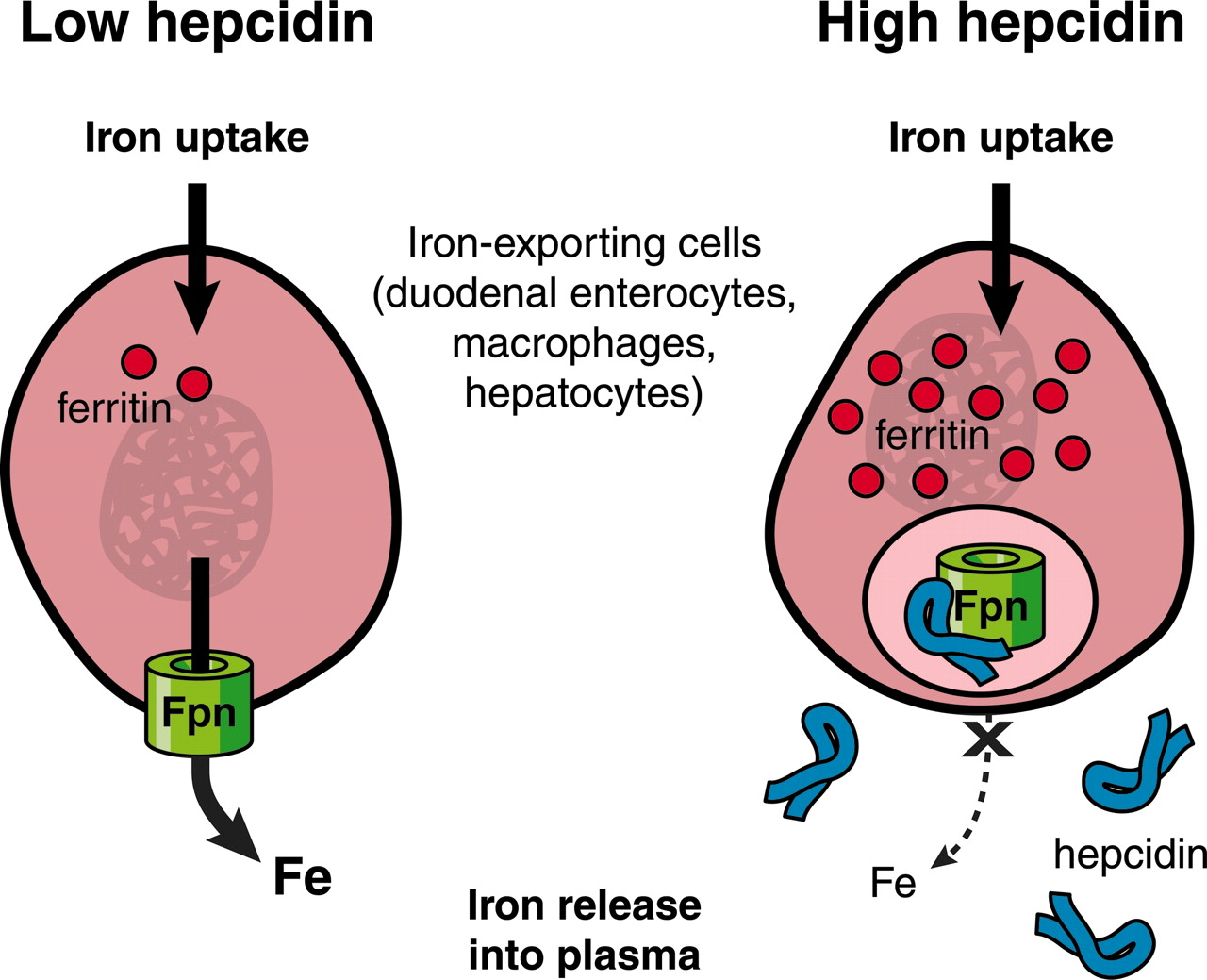
Hepcidin
Binds to ferroportin, inactivating it
Increases when body has adequate Fe stores →decreasing Fe absorption and decreasing release from cells
Decreases when Fe stores drop → Increasing release from cells.
****Hepcidin binds to ferroportin and inactives it. If ferroportin is inactive, there is no way for Fe to get out of the cell. When the body has plenty of Fe, The liver increases hepcidin, decreasing Fe absorption and decreasing the release of of Fe from cells****
Hepcidin Regulation
Involves the Hemochromatosis Gene (HFE)
When there is plenty of iron HFE allows for the production of hepcidin, blocking release of Fe from Storage
Mutation of HFE causes Hereditary Hemochromatosis
EPO role in Hepcidin regulation
Epo enhances a hormone produced by rubriblasts called ERYTHROFERRONE (ERFE) that suppresses hepcidin
Allows more Fe to be released
Apoferritin
Fe 3+ is picked up in blood stream by Apoferritin
✅ Apoferritin binds Fe³⁺ to form ferritin.
📌 Apoferritin binds Fe³⁺ to form ferritin, storing iron in cells & preventing toxicity.
🔹 Key Role in Anemia:
Low ferritin = Iron deficiency anemia;
High ferritin = Inflammatory anemia.
🚀 Key Detail:
Iron is stored in ferritin as Fe³⁺ (ferric form).
Ferrous iron (Fe²⁺) is oxidized to Fe³⁺ before storage.
Transferrin
✅ Transferrin transports Fe³⁺ in the blood to tissues (BM, LIVER, SPLEEN) for storage or utilization.
📌 Key Role in Anemia:
High transferrin = Iron deficiency anemia; Low transferrin = Anemia of chronic disease.
A carrier molecule plus Fe.
Transferrin Receptor 1 (TFR1)
location on RBCs/Retics that Transferrin enters nRBCs
✅ Transferrin Receptor 1 (TFR1) mediates RBC iron uptake by binding transferrin-bound Fe³⁺.
📌 Key Role in Anemia:
Upregulated in iron deficiency anemia to increase iron intake;
downregulated in iron overload.
As cellular iron levels fall the level of ferris decreases fall and TfRs increase
TfRs and cellular iron levels are INVERSELY PROPORTIONAL
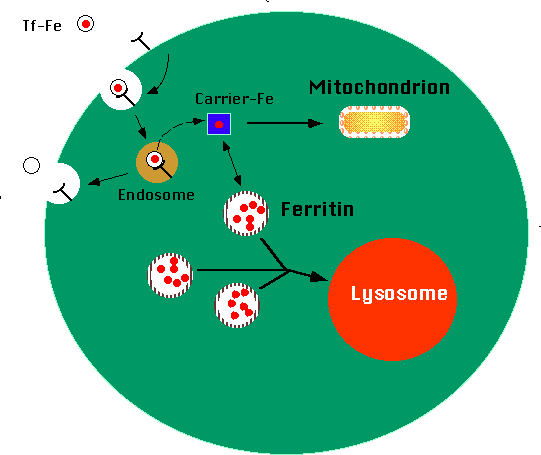
Ferritin
Forms when Apoferritin binds Fe3+
Storage form of Iron in Tissues.
Water soluble- easily mobilized for utilization
equilibrium exists between intracellular stored ferritin and serum ferritin
If serum ferritin is up, Storage ferritin is up AND TFRS FALL
If cellular iron levels fall, the levels of ferritin decrease and the TfRs on cells increase
Serum and Storage Ferritin are DIRECTLY PROPORTIONAL
HEMOSIDERIN
Breakdown product of ferritin found primarily in the RES cells of the LIVER, SPLEEN, and BONE MARROW
Not water soluble
Less readily available than ferritin
Forms a precipitate
Difficult to make usable
Serum Iron
Measure of transferrin bound iron
Fluctuates so should not be used without other labs
Ref Range: Males 65 – 170 μg/dL (usually lower in females)
Serum Iron = Iron bound to transferrin that is in the blood stream.
fluctuates based on diet, very dynamic, so it can’t be examined in solitude
Total Iron Binding Capacity (TIBC)
Total amount of Fe that can be bound to transferrin in serum or plasma
Binding capacity is normally one third saturated
Ref Range: 250-350 μg/dL
Values increase in IDA and decrease in iron overload
The total amount of Iron that can be bound to transferrin (carrier of iron)
The TIBC values are going to increase in Iron def anemia and decreases in iron overload
Transferrin Saturation
% saturation of transferrin is measured as the max amount of iron that is bound in plasma or serum
% Transferrin Saturation = Serum Iron X 100%
TIBC
Ref range: Males: 20-50% Females: 15-50%
Serum Ferritin
Directly proportional to amount of iron stored
Better measure of body storage iron than serum iron and TIBC
Reference Range = 20 – 300 μg/dL
Acute phase reactant
Typical Iron Panel
Serum Iron – Measures the amount of circulating iron bound to transferrin in the blood. It does not directly reflect iron stores in the liver, spleen, or bone marrow. Bone marrow aspiration is typically required for a direct assessment of iron stores.
TIBC (Total Iron-Binding Capacity) – Measures the blood’s ability to bind iron with transferrin, providing an indirect measure of transferrin levels. Higher TIBC suggests iron deficiency, while lower TIBC suggests inflammation or iron overload.
Ferritin – A protein that reflects iron storage in the liver, spleen, and bone marrow. Low in iron deficiency, high in inflammation (acute-phase reactant) or iron overload.
Soluble Transferrin Receptors (sTfRs)
Uncommon Assay
Inversely proportional to amount of iron in the body
Increased when cellular stores of iron are depleted.
They’re not super stable, so they’re easily sloughed off and they go into the blood where they are measured. More helpful in complex cases
Hepcidin levels given Fe stores
Adequate Fe stores–liver increases production of hepcidin – inactivates ferroportin - decreasing Fe absorption and release from cells
When Fe stores drop, decreased Hepcidin produced –less inactivation of ferroportin - increasing Fe absorption and release from cells.
Not widely used. May be used for complex cases of coexisting conditions of IDA and AOI. More than Serum transferrin receptor
Free Erythrocyte Protoporphyrin (FEP) or Zinc Protoporphyrin (ZPP)
Basically, the same test
FEP is the heme without the iron inserted
ZPP is heme with zinc inserted in place of iron
Correlated inversely to ferritin levels
Reticulocyte Count and Reticulocyte Corpuscular Hemoglobin (CHr)
Retic count is a good indicator or RBC production/how BM is responding to anemia
DECREASED with diminished and ineffective erythropoiesis
CHr is an early indicator of iron deficient erythropoiesis.
Good indicator of Pts response to Fe therapy
Bone Marrow Iron evaluation
Rarely justified except in possible sideroblastic anemia, myelodysplatic syndrome or other complex cases.
Usually assessed by serum ferritin but rare cases require BM examination
Iron Deficiency Anemia (IDA)
Basics
Most common anemia worldwide
Epidemiology:
Male: 2%
Female: 5%
Presentation:
Microcytic, Hypochromic RBCs
Small cells are a function of poor Hgb production as are Hypochromic cells.
One of the main components of Hgb is Fe
Causes of Iron deficiency Anemia
Increased Demand
childhood and pregnancy
Excess Loss
menstruation and chronic bleed
Decreased Absorption
gastrectomy & malabsorption syndromes
Iron Poor Diet
milk babies, elderly, adolescence, and pregnancy
**Milk babies = strictly a diet of breast milk past ~4 mons old. Very little Iron
Adolescence bc of poor dietary habits
Iron Deficiency Anemia
Stage 1
IRON DEPLETION
Iron stores in bone marrow are decreased to absent
No decrease in serum Iron
Patients are asymptomatic
Hgb level is normal
RDW usually just slightly elevated
Iron Deficiency Anema
Stage 2
IRON DEFICIENT ERYTHROPOIESIS
Hgb begins to decrease
Hgb contents of retics (CHr) is decreased
HCT is near Normal
Serum Iron decreased
FEP/ZPP increased
If anemia is present
Normochromic, noromocytic cells
Iron Deficiency Anemia
Labs
“CLASSIC IRON DEFICIENCY ANEMIA”
Examine:
Serum iron- low
Serum Ferritin - low
TIBC- High (function of capacity to carry iron. high because they have low iron and have lots of capacity left)
%saturation- low
sTfRs- High
Hepcidin- Low
Marrow Iron- VERY Low
Siderblasts/cytes- Low
M:E ration- More E, so M:E is going to get lower, but since the Retics are destroyed in BM you’l still have a low Retic count.
ZPP- Increased
Iron Deficiency Anemia
CBC Results
Hgb/HCT- Low
MCV- Decreased → Microcytic/Hypochromic RBCs present
MCHC- Decreased
RDW- Increased →Anisocytosis
Poik- Present → Target and Ovalocytes
Retic Count- Decreased
WBC & PLT- Not relevant
Treatment of IDA
Oral Dietary Supplements
If absorption is impaired - IV Iron
Transfusion only in the event of debilitating symptoms or risk of cardiovascular collaspse
Ideally, Retics begin to rise in 4-5 days
Hgb Levels begin to rise in ~weeks (Fully ~6months)
***Not tested on Treatment***
Discuss/List and Explain Two General Mechanisms Involved in Anemic States
1. Increased RBC Loss
Due to hemolysis (RBC destruction) or excessive blood loss.
Can be immune-mediated (DAT+ hemolysis) or non-immune (mechanical, infection, toxins, etc.).
Blood loss may be acute (trauma, surgery) or chronic (GI bleed, heavy menstruation).
2. Decreased RBC Production
Bone marrow failure (aplastic anemia, myelodysplastic syndromes).
Defective erythropoiesis (iron deficiency, B12/folate deficiency, thalassemia).
Anemia of Chronic Inflammation (ACI): Hepcidin-mediated iron sequestration leads to iron unavailability for RBC production.
List Four Hematology Parameters Most Helpful in Evaluating Anemia
1. Hemoglobin (Hgb) & Hematocrit (Hct), RBC
Measures oxygen-carrying capacity of blood.
Low Hgb/Hct = anemia.
2. Mean Corpuscular Volume 2(MCV),
Classifies anemia as microcytic (<80 fL), normocytic (80–100 fL), or macrocytic (>100 fL).
Reticulocyte Count
High retic count: Suggests RBC loss (hemolysis, bleeding).
Low retic count: Suggests decreased RBC production (bone marrow suppression, nutrient deficiency).
NEXT TIER/FOLLOW UP
Serum Ferritin & Iron Studies (TIBC, % Saturation)
Low ferritin = iron deficiency anemia (IDA).
High ferritin with low TIBC = anemia of chronic inflammation.
Look at B-12 & Folate in Macrocytic anemias
Anemia
Reduced oxygen delivery to tissues due to low RBC count, hemoglobin, or both.
Can result from RBC loss, destruction, or decreased production.
Effective Erythropoiesis
Normal RBC production in response to erythropoietin (EPO) stimulation.
Functional bone marrow & adequate iron supply.
Occurs exclusively in the bone marrow
Ineffective Erythropoiesis
RBC production occurs but is defective (e.g., thalassemia, megaloblastic anemia, sideroblastic anemia).
Leads to increased erythroid precursors but decreased mature RBCs.
Primary site of ineffective erythropoiesis due to intramedullary apoptosis of defective erythroid precursors before they enter circulation.
Examples:
Megaloblastic Anemia (B12/Folate Deficiency) → DNA synthesis defects → large, immature precursors (megaloblasts) die in BM.
Thalassemia → Globin chain imbalance → erythroid precursor apoptosis.
Sideroblastic Anemia → Iron-loaded precursors (ringed sideroblasts) fail to mature.
✔ Effective erythropoiesis is primarily in the bone marrow.
✔ Ineffective erythropoiesis occurs mostly in the BM but can involve peripheral destruction.
✔ Liver and spleen may contribute in cases of extramedullary hematopoiesis.
What is the Most Common Cause of Iron Deficiency Anemia (IDA)
Dietary/ nutrient deficiency
menstruation necessitates increased requirements they may nt meet through diet
Anemia of Chronic inflammation (ACI)
Chronic Disease
Secondary to another disease state that has an inflammatory and suppressive effect
Examples:
Chronic Infections
Autoimmune diseases
Infectious mono
Malignancy
Any cause of chronic inflammation
Proposed Mechanisms of ACI
1) Inability of RBCs to access iron trapped within macrophages
Mediated by hepcidin (acute phase reactant, increases with Inflammation)
Increased Hepcidin inactivates Ferroportin and decreases Fe Absorption and release from cells
Iron isn’t getting out of macrophages due to Hepcidin up regulation
Plenty of Iron, but It’s inaccessible.
2) Ineffective level of erythropoietin (EPO)
Cytokines reduce the production of EPO and its responsiveness. Fewer RBCs produced and increased Apoptosis
3) Hemolysis
The inflammatory response overactive the RES (reticuloendothelial system), which may cause damage to RBC membranes and stimulates erythrophagocytosis
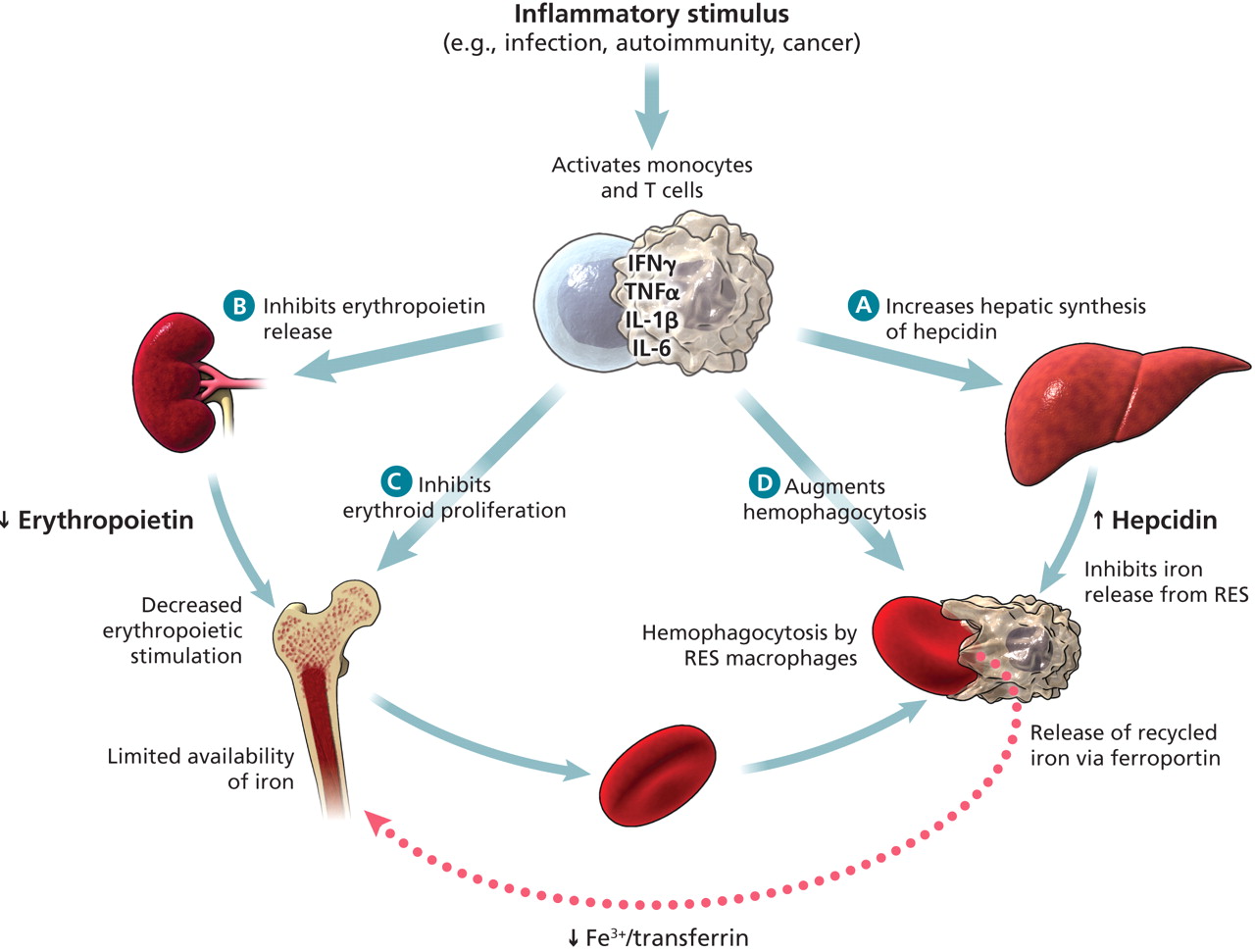
Cytokines & Anemia of Chronic Inflammation
Inflammatory stimulus activates monocytes and T- Cells releasing a flood of cytokines which:
1) Inhibits EPO release
2) Decreased EPO inhibits erythroid proliferation
3) Augments hemophagocytosis
4) Increases hepatic synthesis of hepcidin which inhibits iron release form RES. Also inhibits release of recycled iron via ferroportin.
Key Lab Findings of Anemia of Chronic Inflammation (ACI)
1⃣ MCV (Mean Corpuscular Volume): Slightly low to normal (normocytic or mild microcytic).
Iron is restricted, but not as severely as in iron deficiency anemia (IDA).
2⃣ MCHC (Mean Corpuscular Hemoglobin Concentration): Decreased.
Less hemoglobin per RBC due to impaired iron utilization.
3⃣ Serum Iron: Decreased.
Iron is trapped in macrophages due to hepcidin upregulation.
4⃣ Serum Ferritin: Increased.
Ferritin reflects iron storage, which remains high despite anemia.
Also an acute-phase reactant that increases with inflammation.
5⃣ Bone Marrow Iron Stores: Increased.
Iron is present but inaccessible for RBC production.
6⃣ Sideroblasts (Iron-Stained RBC Precursors in BM): Decreased.
Decreased because Iron is sequestered in Macrophages
7⃣ TIBC (Total Iron Binding Capacity): Decreased.
Transferrin is a negative acute-phase reactant, meaning inflammation reduces its levels.
Less transferrin = less capacity to bind and transport iron.
8⃣ % Transferrin Saturation: Low.
Serum iron is low, and TIBC is also low, but since iron remains trapped in macrophages, saturation is still low.
9⃣ Hepcidin Levels: Increased.
Central cause of ACI—hepcidin blocks iron release from macrophages and enterocytes.
10) ZPP (Zinc Protoporphyrin): Increased.
When iron is unavailable for heme synthesis, zinc replaces iron in protoporphyrin rings.
Marker of functional iron deficiency.
1⃣1⃣ sTfR (Soluble Transferrin Receptor): Normal.
Unlike IDA, iron stores are not depleted, so erythroid precursors do not upregulate transferrin receptors.
1⃣2⃣ Bone Marrow M:E Ratio: Increased.
Myeloid production is unaffected, but erythropoiesis is impaired, increasing the myeloid-to-erythroid (M:E) ratio.
Treatment of ACI
Treat underlying disease
Iron Supplements
EPO stimulating agents
Transfusion only when patient is clinically unstable
Describe the defect(s) in Sideroblastic Anemia
Defect in heme synthesis resulting in “Iron being left at the alter” (Inadequate Fe utilization in heme synthesis)
1) A Hereditary defect in heme synthesis (rare, X- linked def. in aminolevulinic acid synthase)
2) Autosomal recessive trait- results in Stem cell dysfunction:
Decreased heme synthesis
Thrombocytopenia w/abnormal aggregation & prolonged bleeding
Neutropenia- Increased risk of infection
Acquired Defects in Heme Synthesis associated with Sideroblastic Anemia
Acquired Defects in Heme synthesis
Primary:
Myelodysplastic syndromes
Dyserythropoisis/macrocyti
Secondary:
Toxins
Lead poisoning - huge red herring. Associated with cognitive impairment
Drugs
Alcohol
Typical Lab Findings for Sideroblastic Anemia
🔬 Lab Findings:
1⃣ Serum Iron: Normal to Increased – Iron is present but not incorporated into heme.
2⃣ TIBC: Normal to Decreased – No true iron deficiency, iron is not being used properly.
3⃣ Serum Ferritin: Increased – Iron overload from ineffective utilization.
4⃣ sTfRs: Normal to Decreased – Unlike IDA, iron stores are not depleted.
5⃣ FEP (ZPP): Increased – Protoporphyrin accumulates when iron cannot be inserted into heme.
6⃣ Hepcidin: Increased – Iron trapped in mitochondria, but not macrophages like ACI.
Sideroblastic Bone Marrow Findings
🔬 Bone Marrow Findings:
✅ Erythroid Hyperplasia – Ineffective erythropoiesis.
✅ Ringed Sideroblasts (Prussian Blue Stain) – Iron trapped in mitochondria forms a perinuclear ring.
✅ Possible Myeloid/Megakaryocyte Dysplasia – If associated with MDS.
Sideroblastic Anemia Wright Stains/ Peripheral Blood Findings
🩸 Peripheral Blood Smear (Wright Stain):
🔹 Normocytic/Normochromic OR Microcytic/Hypochromic RBCs – Depends on severity.
🔹 Definite Anisocytosis – High RDW.
🔹 Target Cells – Iron dysregulation.
🔹 Basophilic Stippling – Aggregated ribosomes (seen in lead poisoning & some sideroblastic anemias).
🔹 Pappenheimer Bodies – Iron deposits inside RBCs (KEY Clue for Sideroblastic Anemia!).
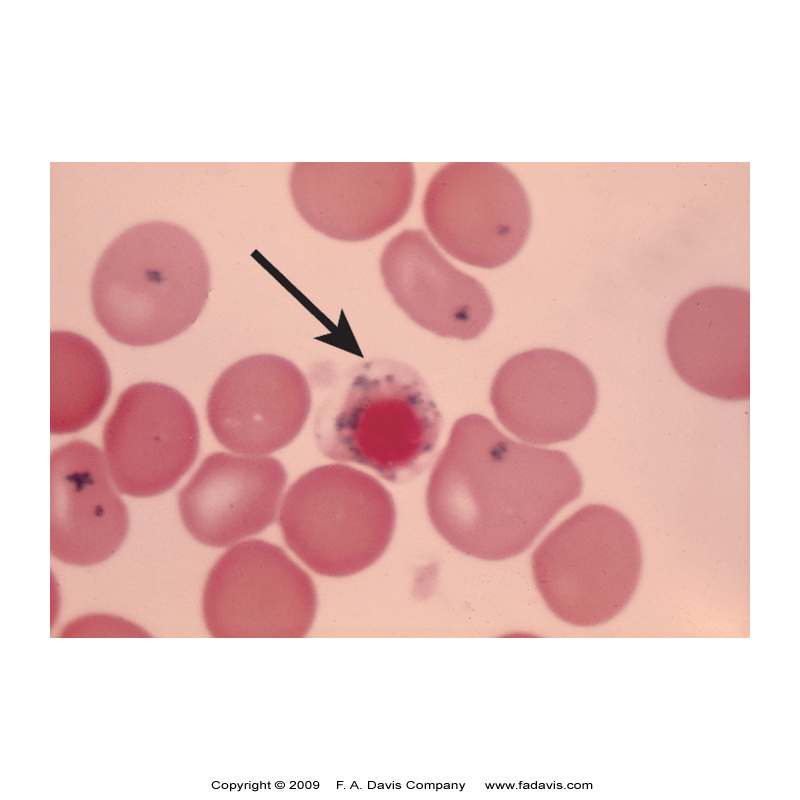
Lead Poisoning and Sideroblastic Anemia
Interferes with Heme Synthesis in at least 3 ways
Inhibits the conversion of:
1) 5-Amino leveling Acid → Porphobilingen
2) Coproporphyrinogen III → Protoporphyrinogen III
3) Protoporphyrin IX —→ Heme
Define Porphyrias
A Group of rare diseases (usually hereditary) that result in errors heme biosynthesis
Caused by a specific enzymatic defect resulting in an accumulation of a specific porphyrin in tissues
Pathological consequences of porphyrias on RBCs
Hemolysis: Porphyrin accumulation causes oxidative damage, leading to RBC destruction.
Ineffective Erythropoiesis: Toxic porphyrins disrupt RBC maturation in the bone marrow.
Photosensitivity: Light-activated porphyrins damage RBCs and surrounding tissues.
Iron Overload: Chronic hemolysis leads to excessive iron deposition.
Anemia: RBC destruction and impaired production result in decreased oxygen transport.
Hemochromatosis
Disorders of Iron Storage
Results in the inappropriate increase in iron absorption leading to excess iron deposited in tissues
Doesn’t typically cause Anemia, but does damage organs (liver, hear, pancreas, joints)
Hereditary Hemochromatosis (HH)
Typically, northern European ancestry (1 in 300 people)
Excessive iron absorption due to Hepcidin deficiency
Bronze skin
Absorb 2-3 times the dietary iron as normal
Type 1 - most common caused by mutation to HFE gene
All 4 types affect Hepcidin, altering regulation of iron absorption
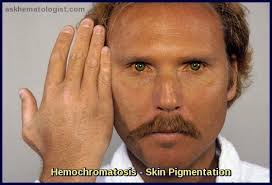
Secondary Hemochromatosis
Acquired or secondary to other inherited anemias and their treatment
Result of repeated transfusions which leads to increased iron storage since the body has no mechanism for iron secretion
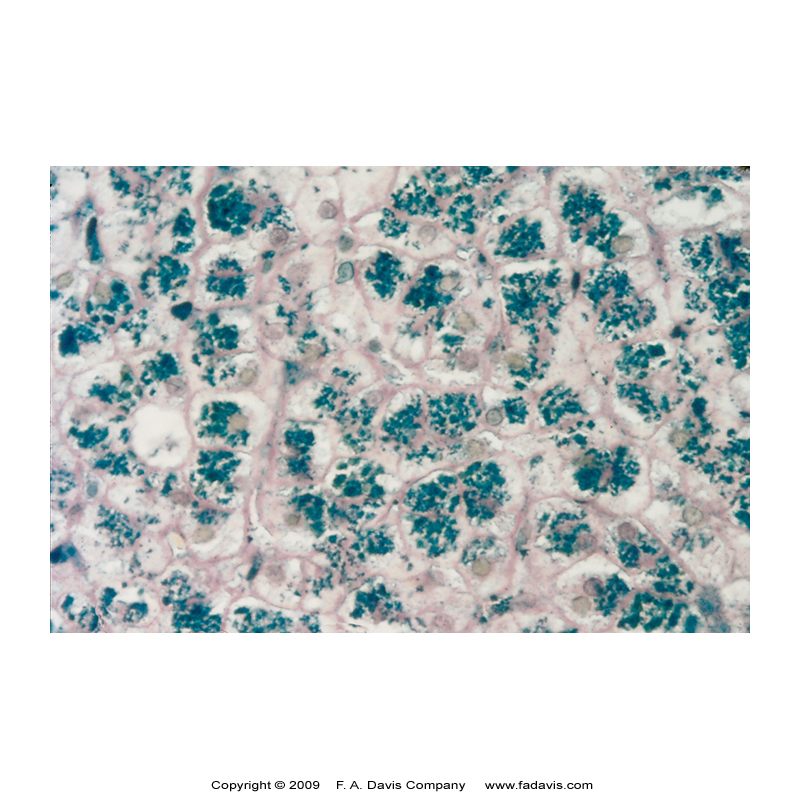
Hemochromatosis Lab Findings & Treatment
Erythropoiesis is normal
Hematologic abnormalities usually not seen
Increased liver enzymes
Iron studies – all increased (TIBC usually normal)
Treatment
Therapeutic phlebotomy and / or chelation therapy
MCHC Formula
(Hgb/HCT) x 100 = MCHC
MCV Formula
(HCT x10/RBC) =MCV
Corrected Retic Formula
reticulocyte count (as %) x (HCT/45) = Corrected Retic
RPI formula
(Corrected Retic/Days to maturation) = RPI
Days to maturation = 45-HCT = x, (x/2) x 0.1 + 1
ex: HCT =30, relic -0.9%
corrected retic = 0.9 s (30/45)=0.667 = 0.6%
Days to maturation, 45-30 = 15, 15/2=7.5, 7.5×0.1=0.75 +1 = 1.75
RPI = 0.6/1.75=0.34 → 0.3%