Seawater Chemistry - Marine Bio
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Density
How much “stuff” is contained in a certain area
Mass divided by volume
Units are grams per milliliter (g/mL)
Density of Water
The density of pure water at 4°C is 1 g/ml
The density of water is affected by certain factors - Temperature - What else is in it (ions in solution)
Temperature
-Water changes density based on temperature in a very unique way.
-Like most substances, water becomes more dense as it gets cooler…
-Except when it gets below 4°C!
Water is most dense at 4 degrees Celsius
What does water do below 4 degrees Celsius
It expands!
As it expands, the water becomes less dense.
When water changes state to ice, it becomes even less dense.
This is why ice floats!
Ions
Many things that are dissolved in water are more dense than pure water.
Therefore, when these are present, water becomes more dense.
Examples: salt, minerals, sugar
Buoyancy
The upward force on an object immersed in a liquid.
Something is buoyant if it is less dense than the liquid that it is in.
Something is not buoyant if it is more dense than the liquid it is in.
Neutrally Buoyant
An object is neutrally buoyant if it has the same density as the liquid it is in.
Something that is exactly neutrally buoyant will neither float or sink.
Fish try to maintain neutral buoyancy
Why do ships float even though steel is more Dense than water?
The air that is contained within a ship, or any cup shaped object, keeps it buoyant.
The density of the air averages with the density of the steel to make the average density of the entire object.
If this average is less than the density of the liquid, then the object floats…
…even if it is open at the top.
Salinity
measure of amount of dissolved salt in a liquid
What happens when a compound dissolves?
breaks up into ions, becomes incorporated into the liquid that surrounds it.
How can salinity be measured
Cause chemical reaction that causes the salts to come out of solution (to un-dissolve)
Measure the conductivity of the water (how easily electricity moves through it)
Boil down the water and find the mass of the leftover salt
How is Salinity Measured?
Salinity is usually measured in parts per thousand (ppt)
The average salinity of seawater in the open ocean is 35 ppt which means that it is 3.5 percent salt.
The average salinity of Narragansett Bay is about 30 ppt (3%)
Why is salinity not consistent everywhere?
Salinity levels fluctuate near land due to freshwater runoff.
In the open ocean, evaporation rates and the formation of ice can change salinity levels.
absorption
Taken out of solution by reacting with other particles that are in the water or on the sea floor
residence time
The average length of time that an ion stays dissolved in seawater
Desalination
removal of salt from seawater
3 methods of desalination
Condensation and Evaporation -Boil water
Reverse Osmosis -Salt water put under pressure against a semi-permeable membrane. The membrane allows only water to pass through. The water that passes through the membrane is fresh
energy
the ability to do work
Heat
Heat is a form of energy measured in calories.
Aside: The Calories on food packages are not the same as calories in science. 1 food Calorie is actually 1 kilocalorie.
Heat is not quite the same thing as temperature.
Specific heat
Specific heat is the amount of heat energy that it takes to raise 1 gram of a substance by 1 degree Celsius.
Water is used as the standard.
The specific heat of water is 1 calorie per gram per degree Celsius (1 cal/g/°C)
Heat Capacity
Heat capacity is the amount of heat needed to raise the temperature of an object by one degree.
Heat capacity is measured in calories/°C
Basically it’s specific heat without dividing by the mass of the object.
With this in mind, if an object has a high specific heat, it also has a high heat capacity.
Heat Capacity of Water
Water has a very high heat capacity.
Water can hold a tremendous amount of heat compared to most other substances.
This is evidenced by the specific heat:
Water 1
Air .25
Soil .1 to .5
(cal/g/°C)
Heat and the Climate
Water’s high heat capacity means that the oceans can absorb a tremendous amount of heat. (Oceans can be referred to as a heat sink)
This allows the oceans to store the heat from the sun and slowly give it.
This keeps the Earth warmer and stabilizes the temperature on Earth.
Local Weather
Similarly, on a local scale, the oceans stabilize the weather of coastal areas.
Coastal areas stay cooler in the summer and warmer in the winter.
Heat affect on organisms
Because the temperature of water rises and falls so slowly, it provides a very stable environment for marine and aquatic organisms.
In one day, the air temperature can change more than 25 °C.
It takes the oceans 6 months to change less than 20 °C
Gasses In Seawater
The three most abundant gases in seawater are nitrogen, oxygen, and carbon dioxide.
Oxygen and carbon dioxide are significant because they are constantly used by organisms.
Exchange of Gases
Oxygen enters seawater in two ways:
Gas exchange with the atmosphere
As the byproduct of photosynthesis by plants and plant-like organisms
Carbon dioxide enters seawater by:
Atmospheric gas exchange
Respiration of organisms
Oxygen Levels
Dissolved CO2 levels do not fluctuate significantly in the Ocean short term
However, oxygen levels are more prone to fluctuation.
This is significant in areas with limited exchange with the open ocean (e.g. estuaries)
Even more significant in fresh water
Eutrophication
Most critical source of oxygen fluctuation
Caused by an excess of nutrients introduced into an area (e.g. nitrogen based compounds like ammonia, nitrites, and nitrates)
Nutrients cause excess growth of microorganisms
Microorganisms consume excess amounts of oxygen
Often the result of fertilizer and/or sewage in runoff
Temperature Pollution
Oxygen levels are also affected by water temperature.
Cooler water can hold more oxygen than warmer water.
Warmer water can hold less oxygen and also speeds up eutrophication.
Temperature and Eutrophication put together
Temperature and eutrophication can work together or separately to cause hypoxia.
Hypoxia: Water condition in which oxygen levels too low to support life.
What are the four types of estuaries based on salinity?
Salt Wedge Estuary, Partially Mixed Estuary, Well-Mixed Estuary, Fjord-Type Estuary
What type of estuary is narr bay
Partially Mixed
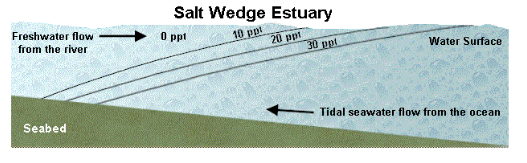
Salt Wedge Estuary
A salt wedge estuary is the most highly stratified type in which there is a strong river flow compared to the mixing that is generated by tidal currents. A distinct freshwater layer flows out over a distinct saltwater layer (or "wedge") and the two are distinct over some length of the estuary.
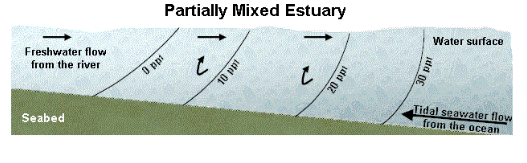
Partially Mixed Estuary
The partially mixed estuary, of which Narragansett Bay is an example, displays a consistent gradient of salinity from the mouth to the head with a corresponding gradient of salinity from the surface to the bottom over a significant length of the estuary.
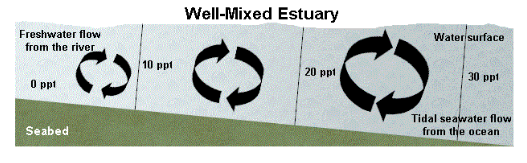
Well mixed Estuary
The well-mixed estuary is dominated by tidal currents over river flow and is thus well mixed over most of its length. While there is a gradient of salinity from the freshwater sources to the mouth, there is little vertical salinity variation, and thus little stratification in this type of estuary.
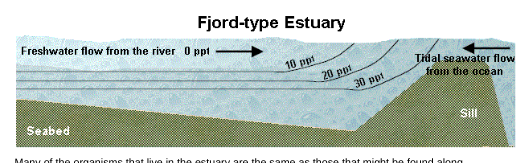
Fjord-type estuary
The fjord is a distinct estuarine type with a different geometry. The typical fjord is relatively long and narrow and is deep along much of its length. A defining feature of a fjord is a sill of shallow water at the mouth, which serves to isolate the deeper waters of the fjord from the ocean. As a result, the deeper waters may not be well mixed, and a fjord can be a highly stratified type of estuary if there is sufficient freshwater.
Besides nitrogen, what other nutrients can be responsible for eutrophication? Where does each of these nutrients tend to become an issue? Why?
Besides nitrogen, the other nutrient that is responsible for eutrophication is phosphorous. Together, nitrogen and phosphorous are what lead in allowing for some aquatic plants, especially algae to grow rapidly and bloom in high densities.
What is the average pH of the oceans? Are the oceans currently acidic?
Over the past 300 million years, the ocean pH has been slightly basic, averaging about 8.2 on the pH scale. Today it's around 8.1.
How can CO2 from the burning of fossil fuels on land end up in the water? In some ways, how is this beneficial?
The oceans currently absorb about a third of human-created CO2 emissions, roughly 22 million tons a day. This has benefited us by slowing the climate change that these emissions would have caused if they had remained in the air.
What are elevated CO2 levels doing to the oceans? Explain briefly.
Due to the high absorption of CO2, continued emissions could reduce ocean pH by another 0.5 units.
How does this affect shelled marine organisms?
Shell-forming animals including corals, oysters, shrimp, lobster, many planktonic organisms, and even some fish species could be gravely affected by the amount of CO2.
If you are a marine organism that doesn’t make a shell, why should you still be concerned?
As the oceans continue to absorb more CO2, their carbon capacity could decrease. This means more of the carbon dioxide we emit will remain in the atmosphere, accelerating global climate change.
What types of organisms are especially prone to death from eutrophic changes in oxygen level?
When dissolved oxygen levels decline to hypoxic levels, fish and other marine animals suffocate. As a result, creatures such as fish, shrimp, and especially immobile bottom dwellers die off due to the changes.
Besides oxygen level changes, how can else can algal blooms from eutrophication be detrimental to other living things?
Algae and other aquatic plant species thrive in conditions that would cause fish to die. This can disrupt entire aquatic ecosystems and their food webs, resulting in loss of habitat and species biodiversity
What is a point source? Give an example of point source eutrophication.
A point source is a single, identifiable, local source of something. Agricultural runoff is often the point source for eutrophication in ponds and lakes.
What is a non-point source? Give an example of non point source eutrophication. Why is this more difficult to fix?
A non-point source is a pollution that cannot be distinguished by itself. It is a cumulative of a small amount of contamination gathering in one area. One nonpoint source is domestic sewage.