Microstructure of plain carbon steels
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
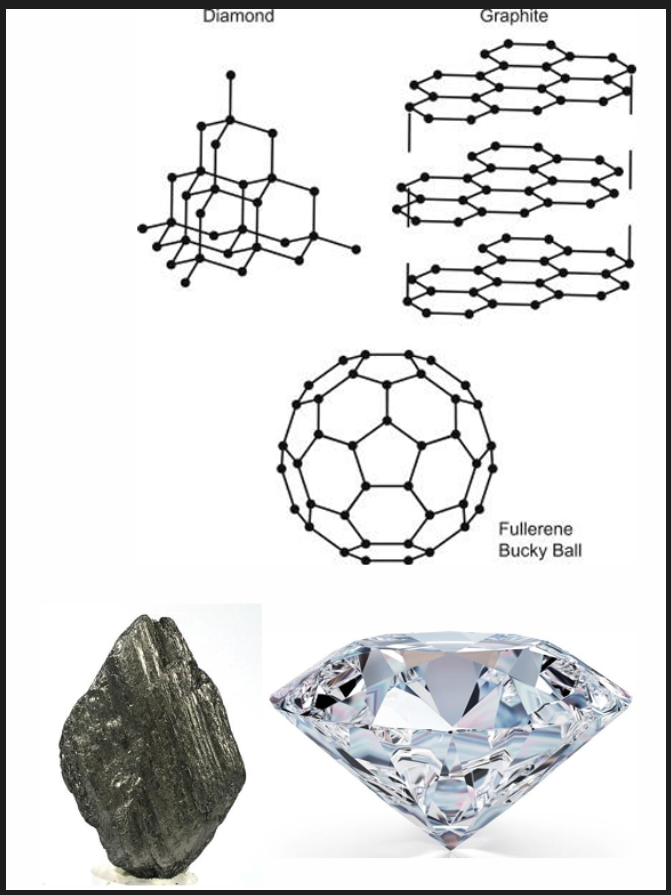
Iron
Allotropic, meaning iron can take different structures/forms. Allotropes of C are diamond, graphite and fullerenes.
Phase changes
Iron changes its crystal structure depending on the temp. Below 910 degrees, iron has a BCC alpha ferrite phase. Between 910 and 1400 degrees, it changes to a FCC gamma austenite phase. Above 1400, it changes back into a BCC phase called a delta phase.
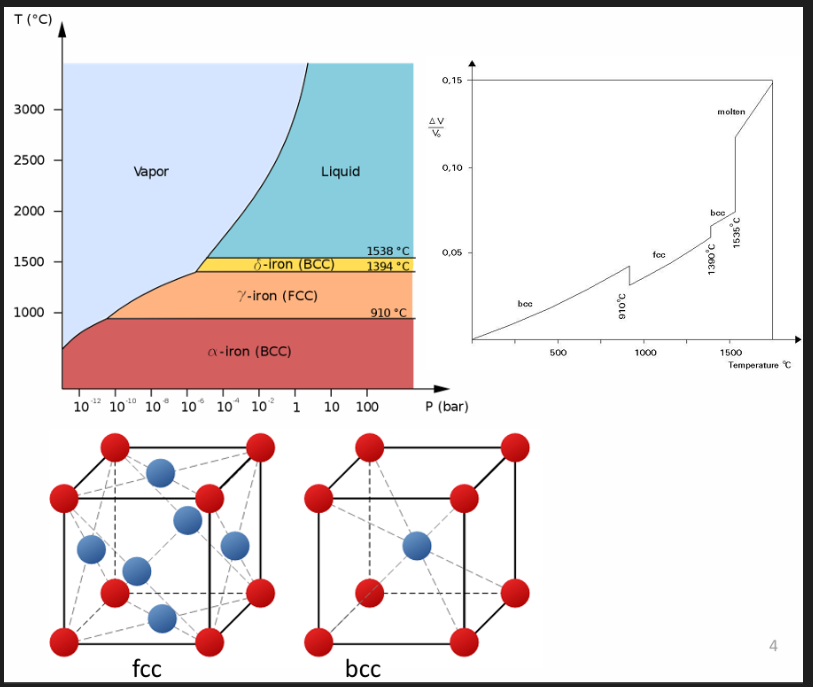
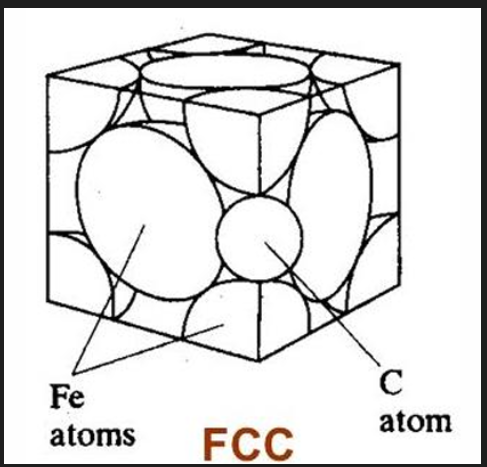
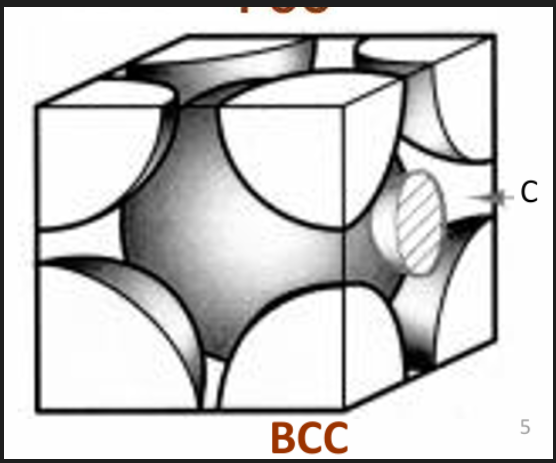
Carbon in iron
C has a higher max. solubility in austenite (FCC, 2.1%) than in ferrite (BCC, 0.025%) due to its crystal structure.
For FCC, C is located at octahedral sites in the centre of each stage
For BCC, C occupies tetrahedral sites. The interstitial sites in BCC iron are smaller than those in FCC iron.
Both are smaller than the C atom itself. But to enter a BCC structure, C will cause more distortion than in an FCC structure, therefore solubility of C in FCC is higher than BCC. More C can fit in the FCC lattice.
Cementite consists of an orthorhombic unit cell with 4 units of Fe3C. Not found as a mineral in nature. Hard (around 800 HV) and brittle. Magnetic up to 215 degrees. Phase diagrams are formed from a family of cooling curves at different compositions.
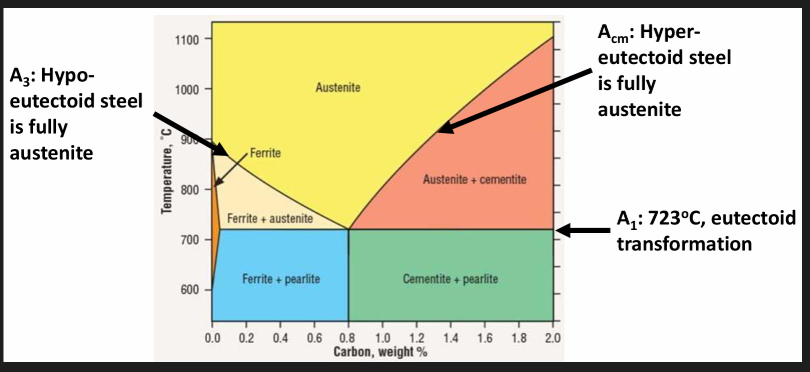
Fe-C phase diagram
Critical change points for steel. see first image.
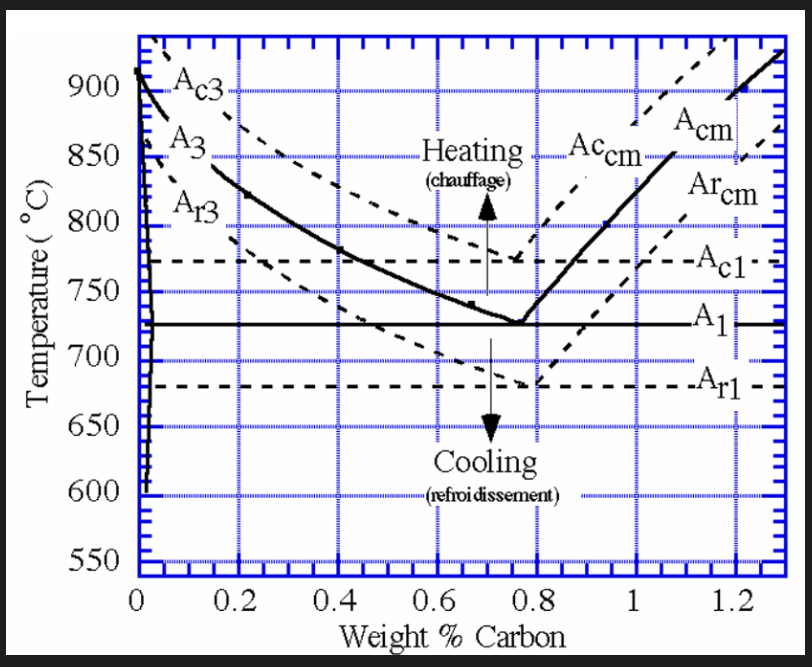
Effect of heating and cooling on critical change points. see second image.
C denotes heating (chauffage)
R denotes cooling (refroidissement)
Tempering heat treatments are conducted by taking a steel at ambient temp. and heating it to the appropriate tempering temp.
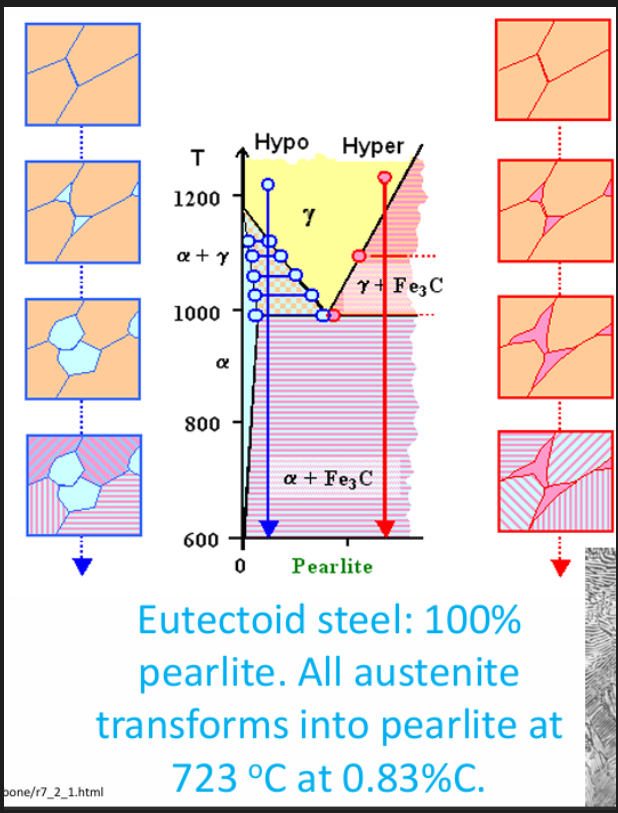
Microstructure of plain C steel
Hypo: proeutectoid (before) and eutectoid (after) ferrite + pearlite
Hyper: proeutectoid (before) and eutectoid (after) cementite + pearlite
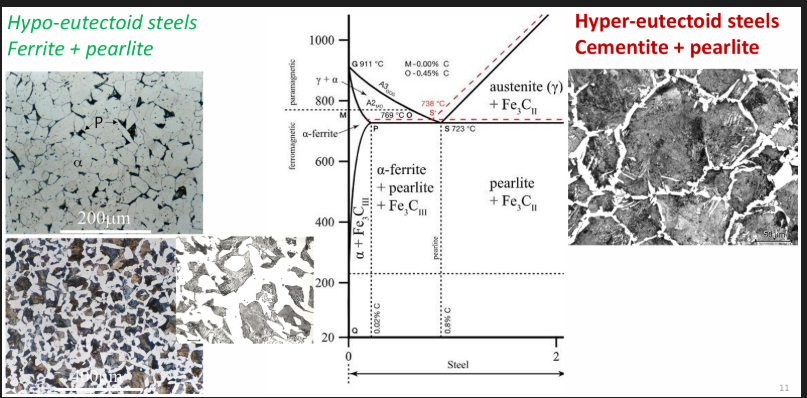
Phases in steels
Ferrite α phase: Iron in BCC form. Has up to 0.006% C at room temp. (increases to 0.025% at 723 degrees). Close to pure iron. Soft, ductile, low strength. Ferromagnetic.
Austenite γ phase: Non magnetic allotrope of iron. Stable above 723 degrees. Can contain higher amounts of dissolved C due to having an FCC structure (up to 2.06% C at 1153 degrees. C in high C steels is limited to about 1.4-1.6%.
Cementite Fe3C: 6.67% C, 93.3% Fe. Metal carbide phase with an orthorhombic crystal structure. Very hard and brittle.
Pearlite: A mix of alternating ferrite and cementite layers. Very tough structure. Thickness of the layers depends on the cooling rate. Contains 0.83% C. In steel, C is combined with Fe (no free carbon, graphite, present in the structure). Max. C content in steel is 2.1%. Above 2.1% C, the extra C will precipitate as free or uncombined flakes or graphite, forming cast iron.
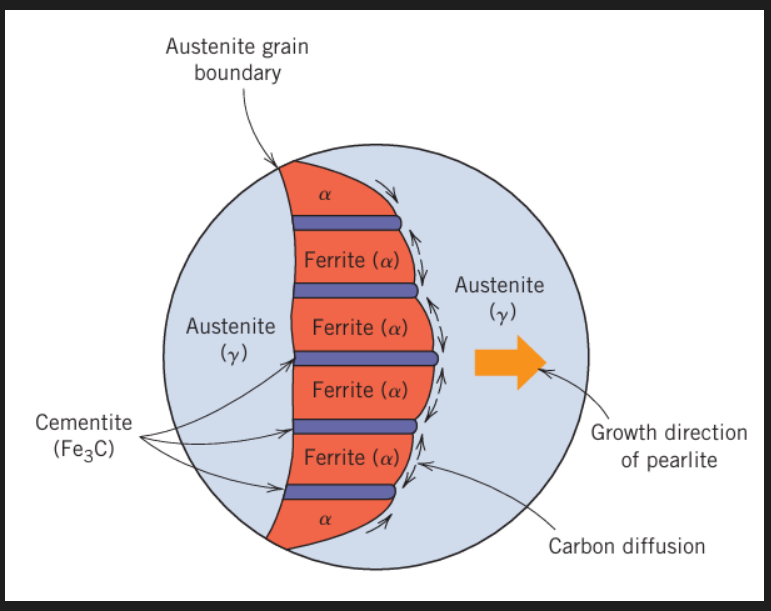
When austenite cools below the eutectoid temp. some ferrite will start growing at the grain boundaries.
This ferrite will expel any excess C in the surrounding matrix until the C percentage is high enough to form Fe3C.
A layer of Fe3C will subsequently form and the process is repeated to get a lamellar structure of alternating layers of alpha Fe and Fe3C.
A lot of solute diffusion will happen in parallel to the growth front within the austenite. This sort of growth is called co operative growth. The distance between the layers of cementite and ferrite is known as the interlamellar spacing.
MnS inclusions
During cooling, the solubility of S in Fe is reduced which results in the formation of FeS. FeS forms at grain boundaries which results in hot shortness. When Mn is added to steel, MnS forms instead. Overly high quantities of MnS affect the mechanical and corrosion properties negatively. MnS reduced friction. Achieve better surface finish. Reduce tool wear when machining steel.
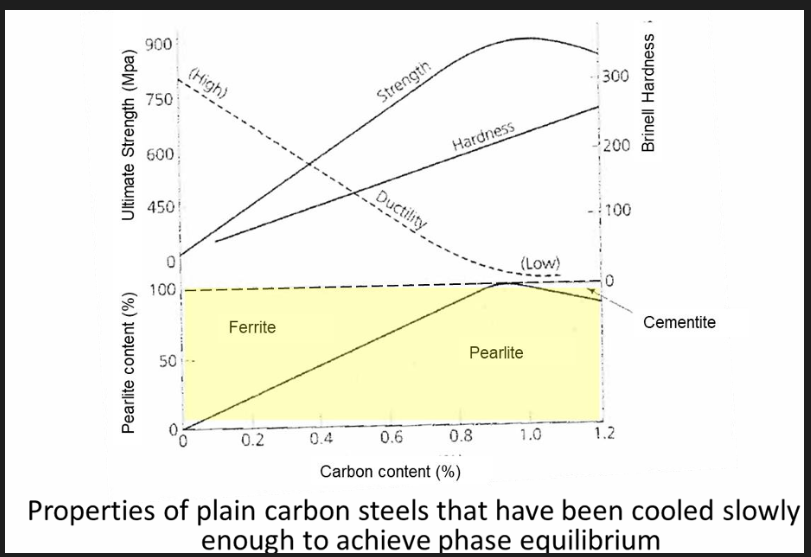
Variation in mechanical properties with %C
Processing
Steel is first cast to get a large ingot. Ingot is then formed (hot worked or cold worked). Heat treatment may then be applied to get the required properties.
Cold working
Metal is formed (plastically deformed) below recrystllisation temp. (usually at room temp.).
Increases strength and hardness
Reduces ductility
Causes strain (work) hardening
Requires higher force than hot working
Examples:
Cold rolling
Wire drawing
Cold forging
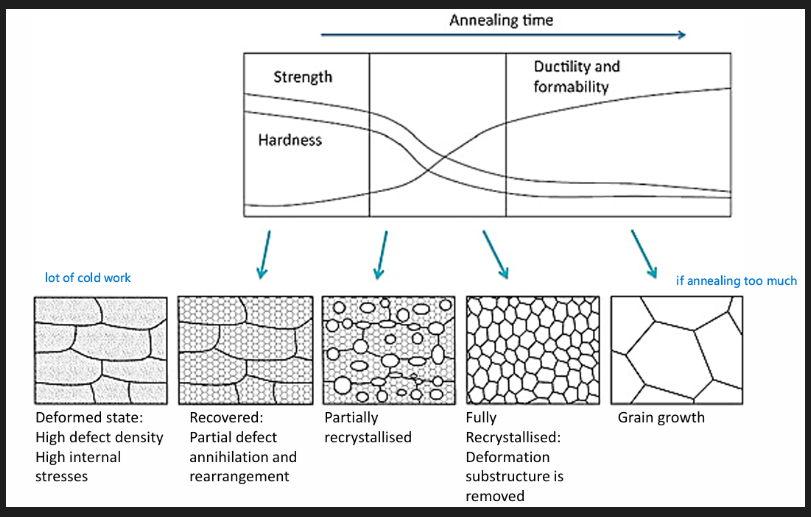
Crystal structure strained and deformed. Metals work harden - becomes stiff and brittle. Work hardening also means a higher forces needs to be applied. To avoid fracture annealing is needed.
Microstructure: see image
Hot working
Metal is formed (mechanical deformation) above recrystallisation temp.
Metal deforms easily with low force
No strain hardening occurs (new grains continuously form)
Improves ductility and toughness
Refines grain structure
Examples:
Hot rolling
Hot forging
Hot extrusion
Dynamic recovery and recrystallisation occur during process. Deformation is not limited by hardening and fracture. Process temp. needs to be limited to avoid burning. Final temp. needs to be low enough to limit further grain growth but high enough to avoid surface cracking.