Shapes of Molecules
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
New cards
What is VSEPR theory?
Lone-pair electrons repel more than bonding pair electrons, so they push bonding pairs closer, making the bond angle smaller.
This minimises lone pair repulsion
2
New cards
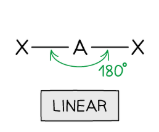
Draw a linear shape
2 bonding, 0 lone (180 degrees)
3
New cards
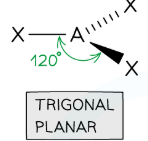
Draw a trigonal planar shape
3 Bonding, 0 Lone (120 degrees)
4
New cards
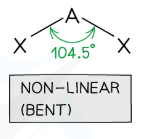
Draw a Bent Shape
2 bonding, 2 lone (104.5 degrees)
5
New cards
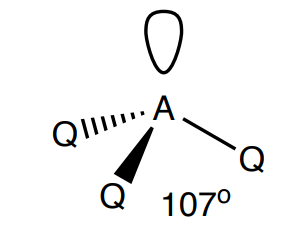
Draw a trigonal pyramidal shape
3 Bonding, 1 lone, (107 degrees)
6
New cards
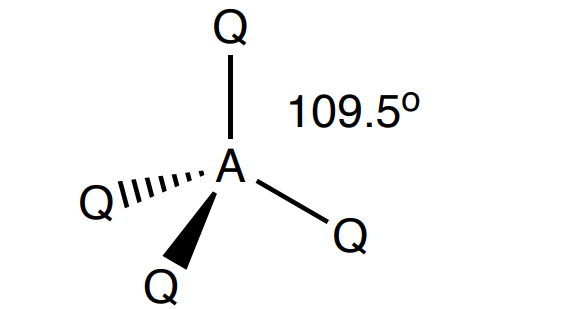
Draw a tetrahedral shape
4 Bonding, 0 lone (109.5 degrees)
7
New cards
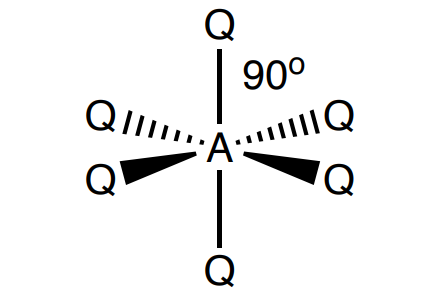
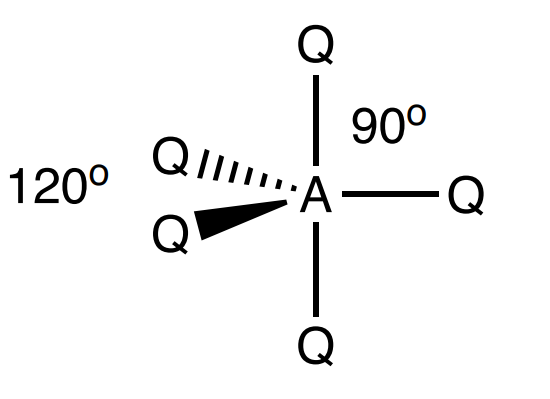
Draw a trigonal bipyramidal shape
5 bonding, 0 lone (90 and 120 degrees)
8
New cards
Draw a octahedral shape
6 bonding, 0 lone (90 degrees)