C-S1M1
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
enantiomers
non-superimposable mirror images
chiral
non-superimposable on their mirror images
achiral
has a plane of symmetry, superimposable onto mirror image
racemic mixture
a 1:1 mixture of enantiomers
How are different conformations of molecules achieved?
rotation around a single bond
stereocentre
carbon with four different substituents attached
Grignard reagent
R-MgX where R is the group being transmuted and X is a halogen
forms new C-C bonds by attacking the carbonyl carbon (carbon with double bond)
What molecule is involved in the second step of a grignard reaction?
H+ attacking O- formed
What are the chiral requirements for making a racemic mixture?
all reactants must be achiral
What structures are achiral?
planar molecules
molecules with carbon with two identical groups attached
In what environment do enantiomers act chemically different?
Chiral
What does a) optically inactive mean and what are b) two types of substances that fall under this category?
a) does not rotate plane polarized light
b) racemic mixtures and achiral molecules
What is the formula for determining max amount of isomers?
2n where n is the number of stereocentres
diastereomers
stereoisomers that are not mirror images
What are the stereochemical relationships between a) enantiomers and b) diastereomers?
a) opposite absolute stereochemistry
b) opposite stereochemistry at not all stereocentres
What kind of molecule will produce all possible stereoisomers?
all stereocenters different
meso compounds
have multiple stereocenters but an internal plane of symmetry, therefore are achiral
TRUE or FALSE: stereocenters are carbons with four different molecules attached
BOTH: while the description is correct, it excludes trigonal planar molecules (with a lone electron pair acting as the fourth group) and non carbon stereocentres
Why are tertiary amines not chiral even if they have four different groups attached?
inversion is too fast due to the low energy barrier therefore enantiomers cannot be separated
allene
has a C=C=C group
atropisomers
stereoisomers resulting from restricted rotation about single bonds
biaryl
two carbon rings joined by a single bond
What affects the possible atropisomers? (2)
need to be able to isolate species (higher energy barrier, half life of at least 1000s)
steric hindrance
resolution
separation of a racemic mixture into its enantiomers
SN1 reaction
nucleophilic substitution with Rate = k [alkyl halide], forming carbocation intermediate
![<p>nucleophilic substitution with Rate = k [alkyl halide], forming carbocation intermediate</p>](https://knowt-user-attachments.s3.amazonaws.com/b582daec-1449-4355-aeee-559dfefa4fc1.png)
SN2 reaction
nucleophilic substitution with Rate = k [alkyl halide] [nucleophile]
![<p>nucleophilic substitution with Rate = k [alkyl halide] [nucleophile]</p>](https://knowt-user-attachments.s3.amazonaws.com/f5575314-aecf-4a5b-8210-1f1c2a665ba3.png)
What determines whether a substrate will react via SN1 or SN2?
stability of carbocation (tertiary), ability of nucleophile to approach from opposite leaving group (primary)
What are methods enantiomers can be separated? (4)
covalent bond formation
salt formation
chiral chromatography
enzymatic resolution
What is the main limitation of all resolution techniques?
Lose half of your product
What SN reaction do a) primary, b) secondary, c) tertiary substances form?
a) SN2
b) either but not very well (loser ahh), more likely SN2 though but depends on circumstances
c) SN1
What shape do carbocations form?
can only be planar
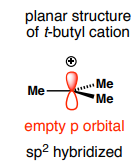
How does the planar shape of carbocations impact nucleophilic substitution?
Alkyl substituents can stabilize a carbocation through electron donation when C-H or C-C bonds are parallel to the empty p orbital → forms stable tertiary carbocations for SN1, nucleophile can attack from either plane
What is the stereochemistry of products of a a) SN1 and b) SN2 reaction?
a) racemic mixture
b) inversion (eg. R → S)
What are two reasons methyl and primary alkyl substrates react by SN2?
the carbocation is very unstable
low steric interference allowing the nucleophile to attack from opposite the leaving group
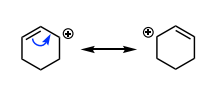
TRUE OR FALSE: Conjugation allows for more products
TRUE, via delocalization of the carbocation/ resonance stabilization of the carbocation allowing more reaction sites (can make a non-tertiary carbocation for SN1)
What makes/are a) excellent, b) good, and c) poor leaving groups?
a) neutral, partial negative charge from lone pair of electrons, single weak bond to carbon; -H2O, -ROH, -NH3, -RNH2, -R2NH, -H2S, -RSH
b) stabilized charge; Br- (halogens), RCO2-, RSO3
c) unstabilized charge eg. RO-, RN-H, RS-
How can pKa be used to determine the strength of a leaving group?
Lower pKa = stronger acid = more stable conjugate base = better leaving group
How can OH, a bad leaving group, be made to leave? (3)
protonation with acid to make H2O
combination of O with S of P (strong bonds to O)
use of mesylate (MsCl) or tosylate (TsCl) for primary or secondary alcohols; better leaving groups= ROTs/Ms to replace it
How can an ether (R-O-R) be made to react and why does it not usually do so?
they are very stable but can be activated by a protic acid (H from acid joins the lone pair on O, SN2 addition of conjugate base to R leaving group) or Lewis acid
epoxide
highly strained three membered ring
How can ring strain of epoxides be released?
SN2 ring opening via amine RNH2 nucleophiles
OR H2O to give 2 OH groups
What makes a a) weak, b) moderate and c) strong nucleophile?
a) neutral, partial negative charge from lone pair of electrons eg. H2Ö: . single weak bond to carbon
b) stabilized charge eg. Br-
c) unstabilized charge eg. RO-
*same groups as leaving group but opposite strength
What type of nucleophile favours an a) SN1 and b) SN2 reaction?
a) weak
b) strong
Why are reactions between NH3 and alkyl halides unselective?
the primary amine product is at least as nucleophilic as NH3, so it can also react with the alkyl halide
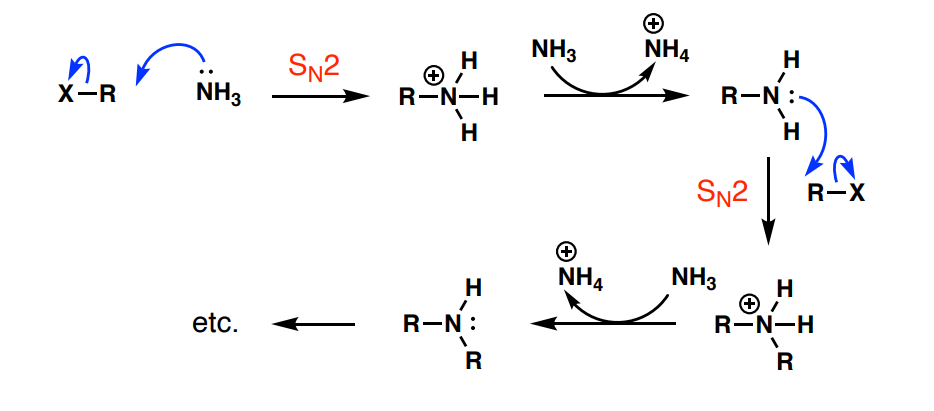
How would an amine (NH2) be added to an alkyl halide in a nucleophilic substitution?
R-X + NaN3 → R-N3
R-N3 —LiAlH4→ R-NH2
TRUE OR FALSE: both SN1 and SN2 reactions require a polar solvent
TRUE
What kind of solvent favours a) SN1 and b) SN2 reactions?
a) polar protic
b) polar aprotic
What is the use of solvent in a) SN1 and b) SN2 reactions?
a) stabilize carbocation intermediate
b) dissolve nucleophile
What is a a) polar protic solvent and b) what are some common examples (3)?
a) has hydrogen attached to an electronegative atom ie. O or N
b) H2O, MeOH, EtOH
What is a a) polar aprotic solvent and b) what are some common examples (4)?
a) no protons attached to electronegative atoms
b) DME, acetone, DMSO, DMF
Why is polar aprotic better for SN2?
Polar aprotic solvents do not form strong solvent shells around nucleophiles, allowing the nucleophile to attack unhindred
TRUE OR FALSE: rotation around a single bond produces a diastereomer of the molecule
FALSE: it is the same atom! just a different conformation
What is the difference between a configuration and a conformation?
configurations are stereoisomers and bonds need to be broken to interconvert them, conformations are the same molecule and can be interconverted by rotation around a single bond
Which conformation of a Newman projection is a) higher in energy and b) lower in energy?
a) eclipsed
b) staggered
torsional strain
a property of conformations in which the dihedral angle between adjacent bonds is other than 60° (in which case it is 0)
Why do eclipsed bonds produce more torsional strain?
electron repulsion and steric interference
What is the energy range for the barriers for rotations about most single bonds?
12-21 kJ/mol
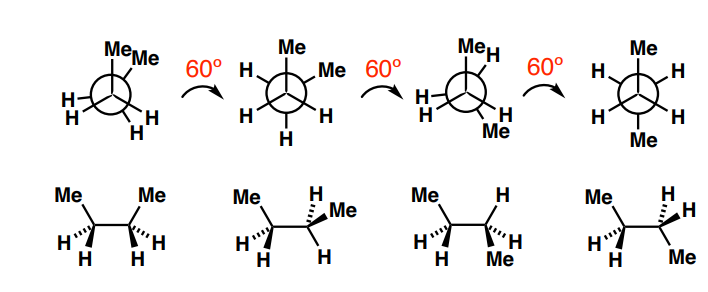
Name the conformations of the methyl groups (1 to 4 left to right)
syn-periplanar: same side, same plane
gauche: at 60o
anticlinal
anti-periplanar: opposite side, same plane
conformational isomers/conformers
3 most stable conformations (lowest energy minima) of a molecule that will be isolated; 2 gauche, 1 anti-periplanar
a) What cycloalkane has the lowest ring strain and b) how low is it?
a) cyclohexane
b) 0
What are a) the two types of substituent in cyclohexane and b) which one is more stable and c) why?
a) axial (sticking out in plane) and equatorial (up and down from points)
b) equatorial
c) no diaxial interactions between axial substituents
What determines the favoured conformation of cyclohexanes?
max groups equatorial, or if equal groups, largest equatorial
How can ring inversion be stopped?
at low temperatures, the conformations interconvert more slowly, HOWEVER, to permanently lock it, one must use t-Butyl (—C(CH3)3) which is bulky hence must be equatorial, or another ring which can be either axial or equatorial
What conformer of cyclohexane will SN2 reactions favour in regards to the leaving group and why?
axial leaving group as there is space on opposite side
epimer
diastereomers that differ in configuration at only one chiral centre (more typically for compounds with more than two stereocenters)
What makes allenes chiral as opposed to achiral?
Cannot have two of the same same substituents at any ends (minimum of two different substituents with one of each at each end)
Cahn-Ingold-Prelog rules
highest atomic number= 1, lowest = 4
draw molecule with the lowest priority substituent (priority 4) at the rear
assign R or S based on 1→2→3
What elimination mechanism resembles the SN1 reaction in terms of mechanism?
E1
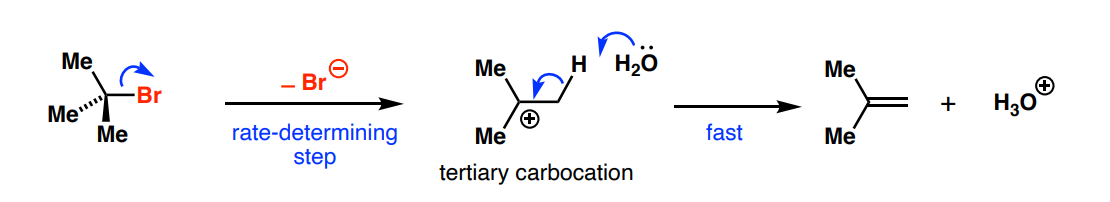
What halogen makes the best leaving group in an E1 reaction?
I
What are the two considerations for the stereoisomer formed from E1?
To form new C=C double bond, the C-H bond that’s broken must be in same plane as empty p orbital (ie. H in eclipsed position to empty p orbital)
The carbocation conformation where the two largest groups are furthest apart is lower in energy therefore favoured
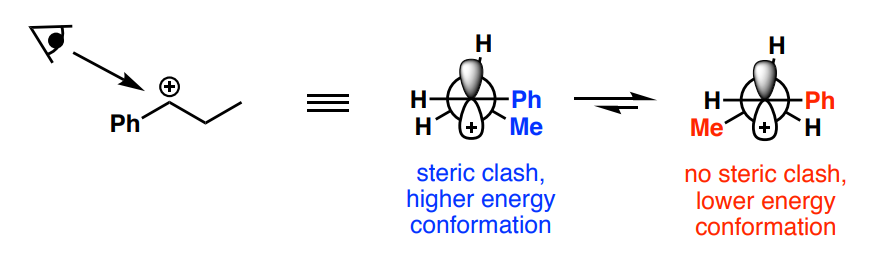
What elimination mechanism resembles the SN2 reaction in terms of mechanism?
E2
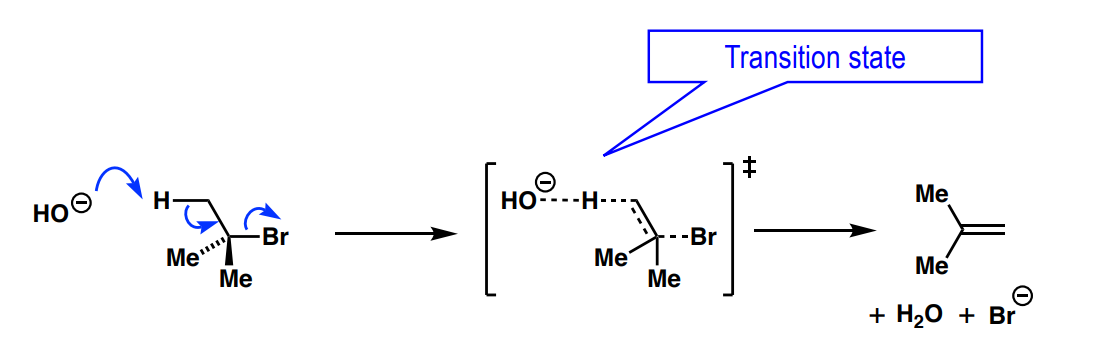
For E2, what must the relationship be in a) a Newman projection between the two atoms eliminated (X and H) and b) which is predominant?
a) syn-periplanar or anti-periplanar
b) anti-periplanar / anti-elimination
E2 is not affected by (substitution/base), E1 is not affected by (substitution/base)
E2 is not affected by (substitution/base), E1 is not affected by (substitution/base)
What is the pKa of a conjugate acid of a) weak b) moderate c) strong bases?
a) <3
b) 3-12
c) >12
Does a) E1 and b) E2 favour a strong or weak base?
a) weak
b) strong
a) Which elimination/substitution mechanism is more sensitive to the nature of the leaving group and thus b) which will a good, not excellent, leaving group prefer?
a) E1/SN1
b) E2/SN2
The (less/more) substituted alkene is formed in greater amount
more
Do higher temperatures favour elimination or substitution?
elimination
What reaction forms an alkene?
elimination
How can we tell whether a reagent will act preferentially as a nucleophile or a base? (4)
Charge density (stabilisation) plays a part – The lower the charge density, the poorer the base
Charge stabilisation through size or resonance – Nucleophilicity is less affected by charge density
A reagent with a partial negative charge (e.g. from a lone pair of electrons) will usually (but not always) be a better nucleophile than a base
Steric factors of substrate and nucleophile – Can affect the ability of a reagent to act as a nucleophile (e.g. t-BuO–), but less important for bases