Conformations (Chapter 3)
0.0(0)
Card Sorting
1/14
Earn XP
Description and Tags
Last updated 7:57 PM on 9/23/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
1
New cards
conformations
different rotational arrangements of molecules
2
New cards
conformer
a conformation with local energy minimum
3
New cards
When do molecules experience strain?
When they are forced to adopt a non-ideal condition (not be in the lowest possible energy state)
4
New cards
angle strain
bond angles deviating from their ideal values
5
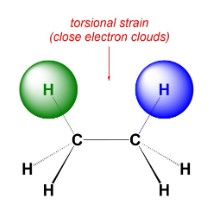
New cards
torsional strain
electron clouds from two atoms that are seperated by exactly three bonds come close together
6
New cards
steric strain
two electron clouds overlap (a specific number of bonds is NOT required)
7
New cards
eclipsed conformation
the substituents on two atoms are directly in front of each other in a Newman Projection
8
New cards
staggered conformation
the substituents on two atoms are the maximum distance from each other in a Newman Projection (lowest energy conformation)
9
New cards
What is the most common reason for angle strain in a molecule?
geometric constraints
10
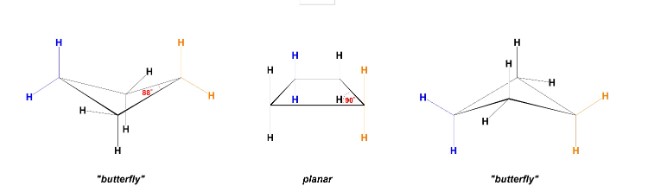
New cards
What type of conformers can be made by 4-membered rings?
butterfly conformers
11
New cards
ring inversion (ring flip)
changing between the lowest energy conformations of a ring
12
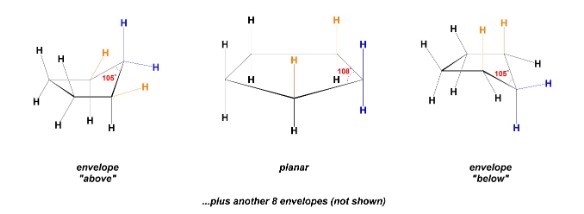
New cards
What type of conformers can be made by 5-membered rings?
envelope conformers
13
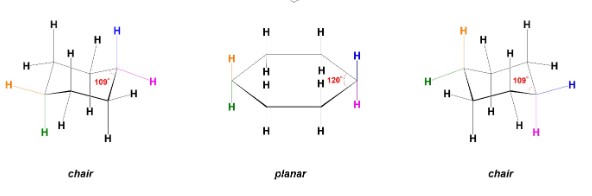
New cards
What is the most stable conformation for 6-membered rings?
chair conformations
14
New cards
axial groups
the 6 substituents that align with the z-axis (up and down)
15
New cards
equatorial groups
the 6 substituents that point towards the mid plane of the ring