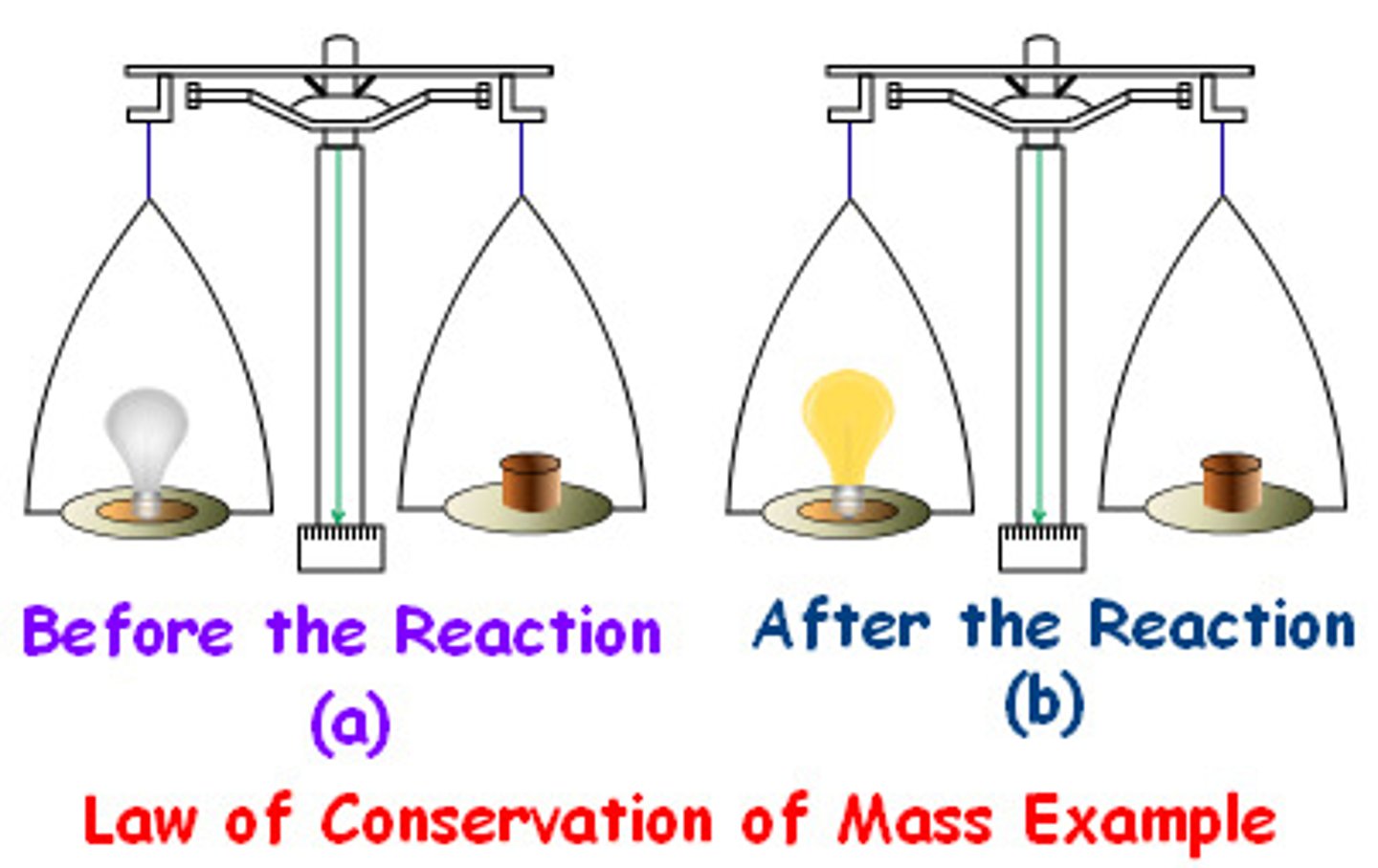
Law of Conservation of Mass
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Law of Conservation
Matter is neither created nor destroyed
Chemical Equation
a way to describe a chemical reaction using chemical formulas and other symbols
Reactant
A chemical substance that is present at the start of a chemical reaction
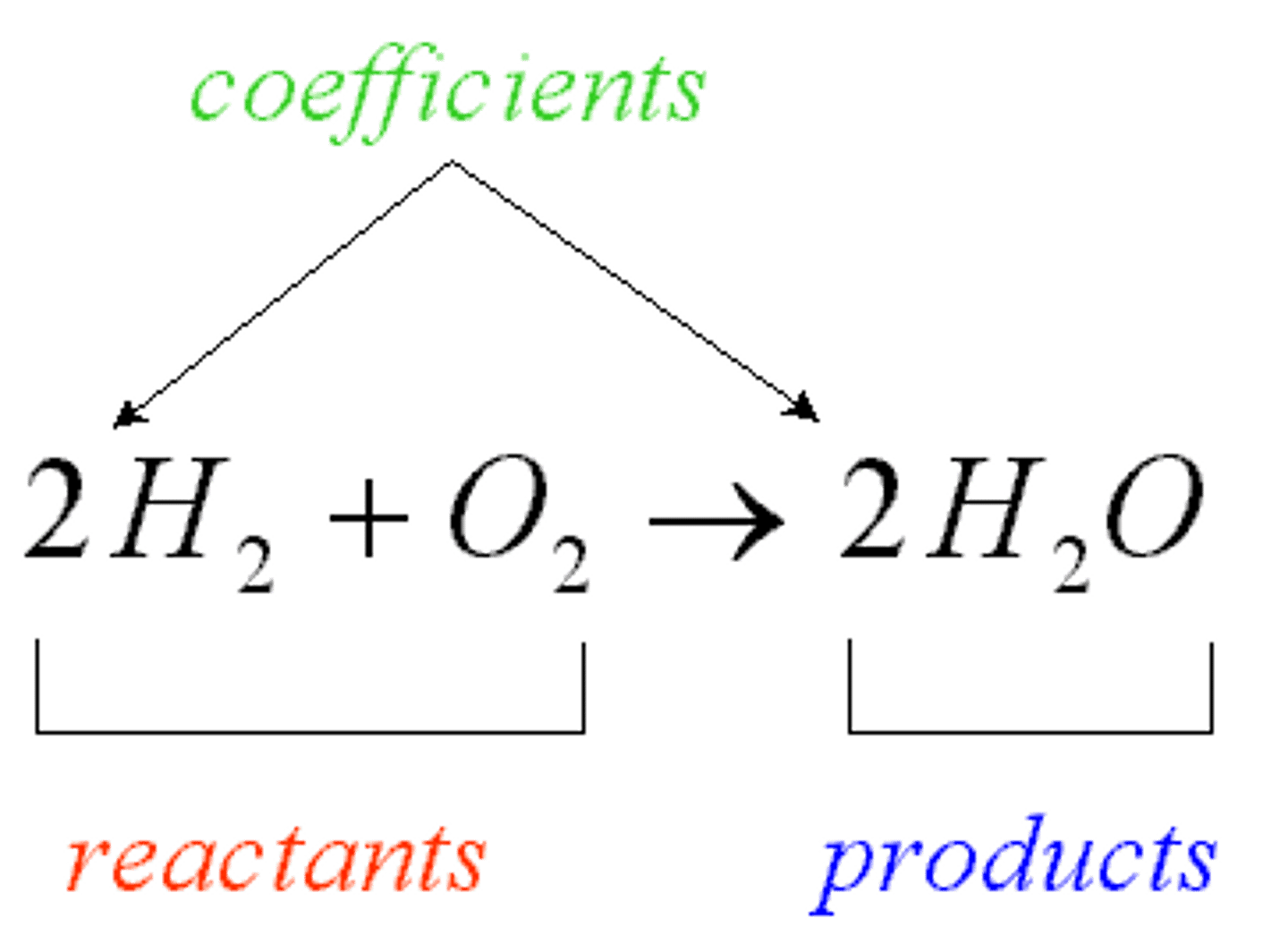
Product
A chemical substance formed as a result of a chemical reaction

balanced equation
We have the same amount and kind of atoms in the reactants and in the products
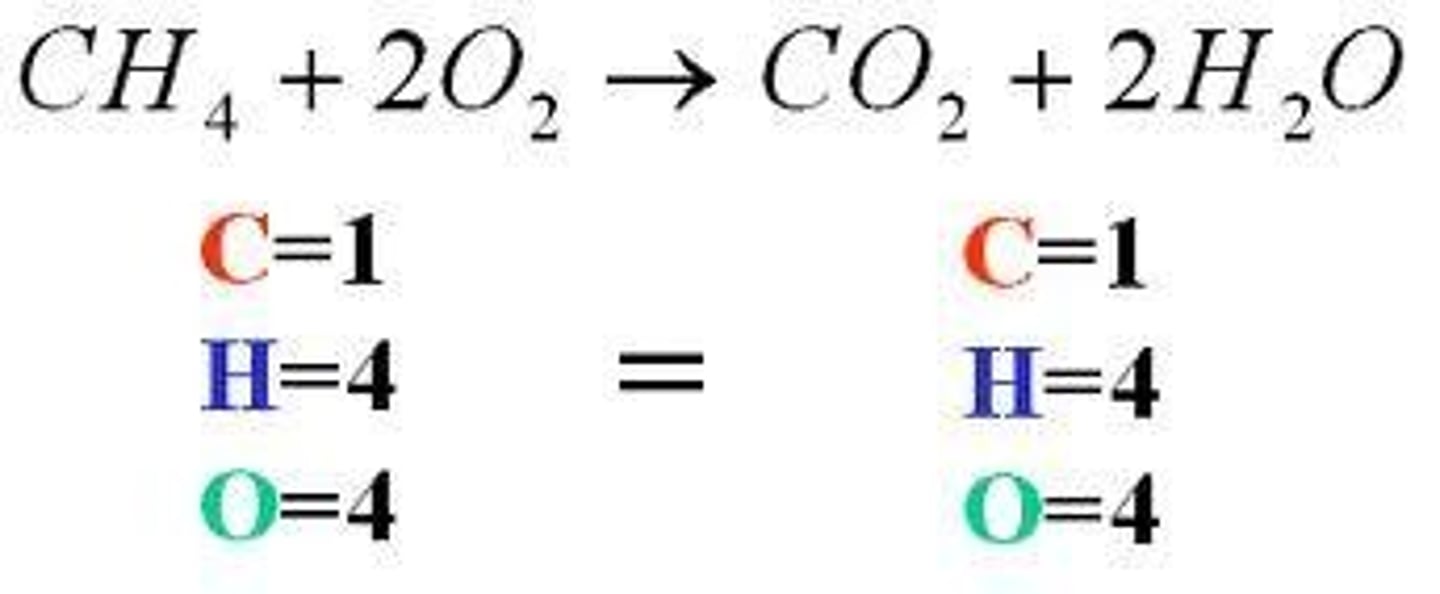
Chemical Reaction
A reaction that changes the original substances. A new substance is form. Cannot be reversed.

Physical Reaction
A reaction that occurs without changing the original substances. NO new substance is formed. It can be reversed.

Examples of Chemical Reactions
Rust, oxidation,milk and vinegar, glows ticks

What elements are in the reactant side if we have a reaction products of NaCl
Na- sodium and
Cl - chlorine
What do you call the stuff you end up with in a chemical reaction?
products
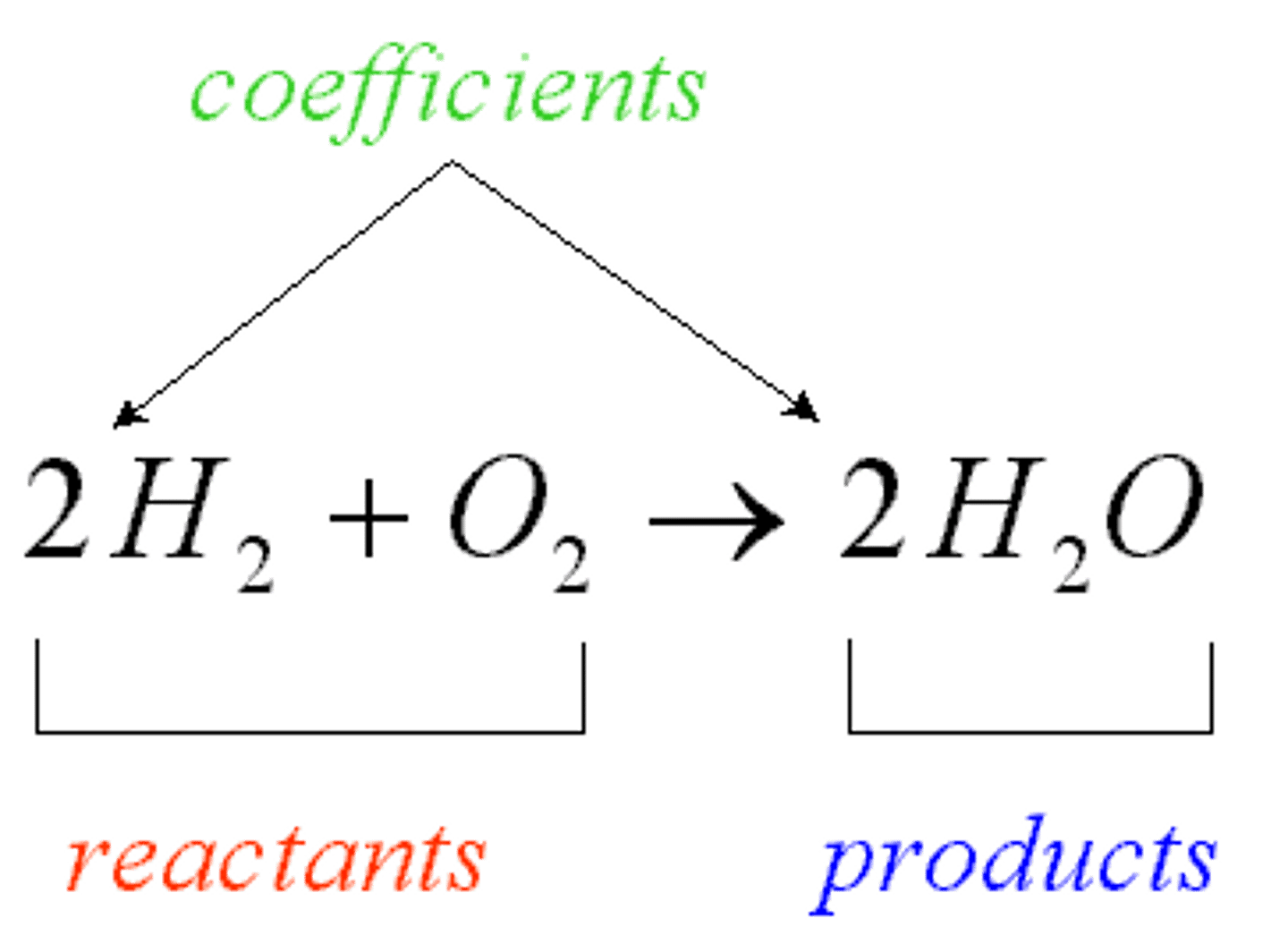
should you have the same amount of atoms at the beginning and at the end of the reaction?
yes
Conservation of Mass
mass of the legos 50g - rearrange - mass of the legos 50g