Biomolecules
How to analyze Chemical Composition?
We can take any living tissue (a vegetable or a piece of liver, etc.) and grind it in %%trichloroacetic acid (Cl3CCOOH)%% using a mortar and a pestle.
- We obtain a thick slurry.
- If we were to strain this through a cheesecloth or cotton we would obtain two fractions.
- One is called the filtrate or more technically, the acid-soluble pool, and the second is the retentate or the acid-insoluble fraction.
- Scientists have found thousands of organic compounds in the acid-soluble pool.
Analytical techniques, when applied to the compound give us an idea of the molecular formula and the probable structure of the compound.
All the carbon compounds that we get from living tissues can be called ‘biomolecules’.
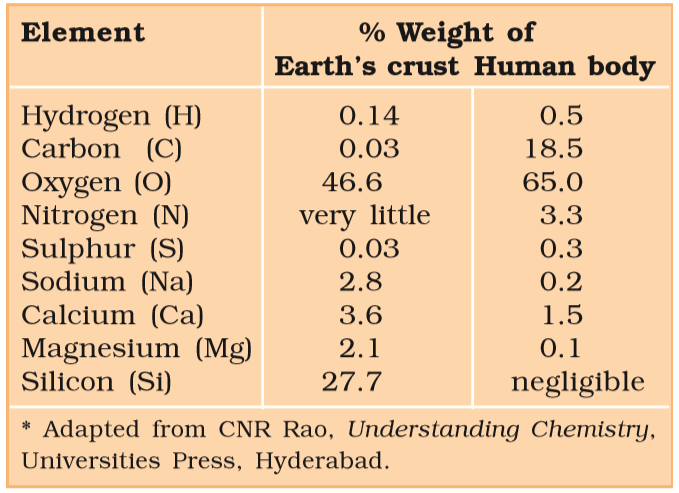
- However, living organisms have also got inorganic elements and compounds in them.
A slightly different but destructive experiment has to be done.
- One weighs a small amount of living tissue (say a leaf or liver and this is called wet weight) and dries it.
- All the water evaporates.
- The remaining material gives dry weight.
- Now if the tissue is fully burnt, all the carbon compounds are oxidized to gaseous form (CO2, water vapor) and are removed.
- What is remaining is called ‘ash’.
- %%This ash contains inorganic elements (like calcium, magnesium, etc).%%
- %%Inorganic compounds like sulfate, phosphate, etc., are also seen in the acid-soluble fraction.%%
- Therefore elemental analysis gives the elemental composition of living tissues in the form of hydrogen, oxygen, chlorine, carbon, etc. while analysis for compounds gives an idea of the kind of organic and inorganic constituents present in living tissues.
- From a chemistry point of view, one can identify functional groups like aldehydes, ketones, aromatic compounds, etc.
- But from a biological point of view, we shall classify them into amino acids, nucleotide bases, fatty acids, etc.
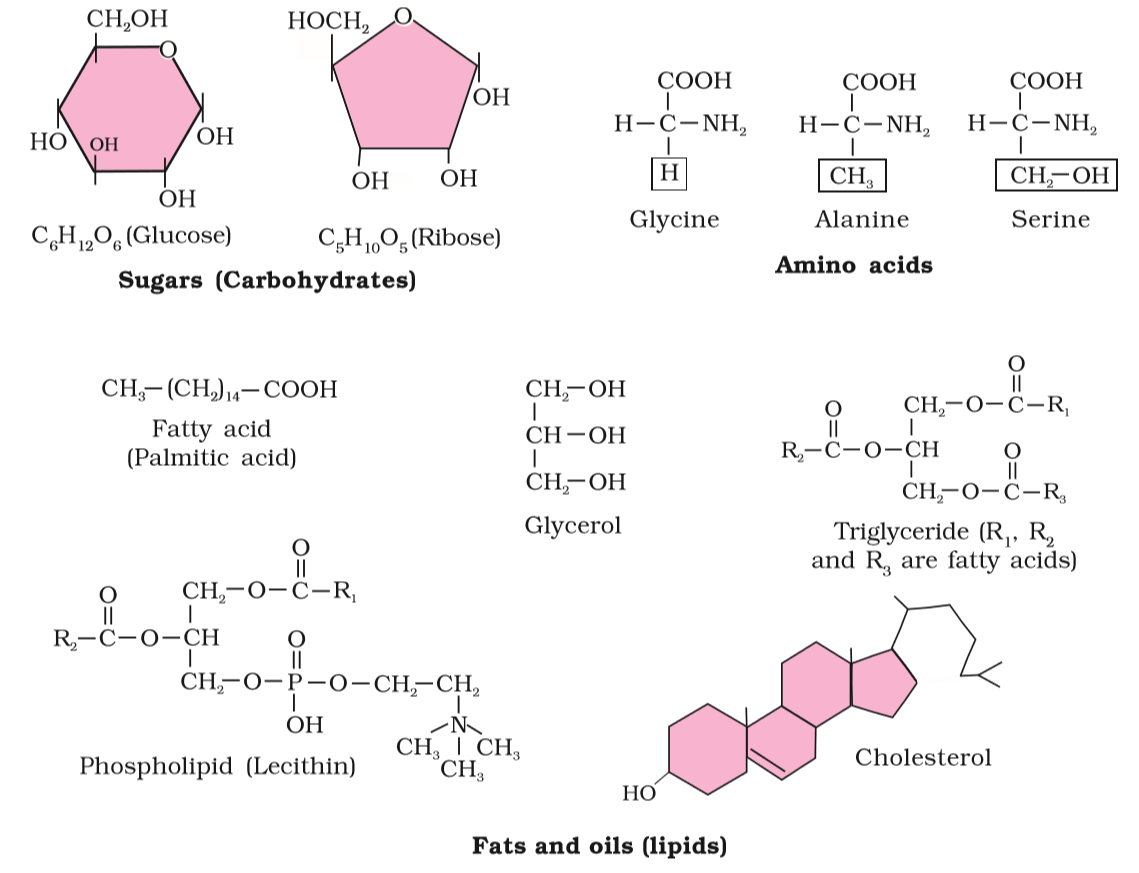
Amino acids are organic compounds containing an amino group and an acidic group as substituents on the same carbon i.e., the α-carbon.
- Hence, they are called α-amino acids.
- They have substituted methanes.
- There are four substituent groups occupying the four valency positions.
- These are ^^hydrogen, carboxyl group, amino group, and a variable group designated as the R group.^^
- Based on the nature of the R group there are many amino acids.
- However, those which occur in proteins are only twenty types.
- The R group in these proteinaceous amino acids could be ^^hydrogen (the amino acid is called glycine), methyl group (alanine), hydroxy methyl (serine), etc.^^
- The chemical and physical properties of amino acids are essential to the amino, carboxyl, and R functional groups.
- Based on a number of amino and carboxyl groups, there are
- acidic (e.g., glutamic acid)
- basic (lysine)
- neutral (valine) amino acids.
- Similarly, there are aromatic amino acids (tyrosine, phenylalanine, tryptophan).
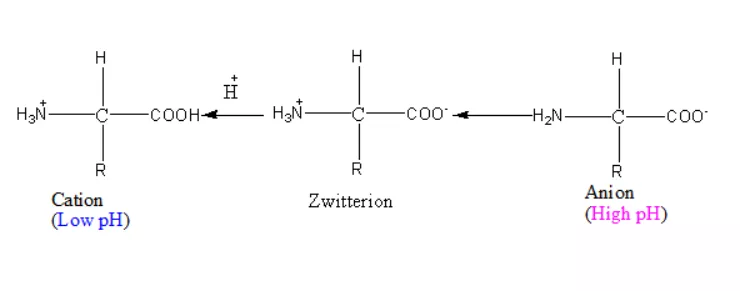
- A particular property of amino acids is the ionizable nature of –NH2 and –COOH groups.
- Hence in solutions of different pH, the structure of amino acids changes
Lipids are generally water-insoluble.
- They could be simple fatty acids.
- A fatty acid has a carboxyl group attached to an R group.
- The R group could be a methyl (–CH3 ), ethyl (–C2H5 ), or a higher number of –CH2 groups (1 carbon to 19 carbons).
- For example, palmitic acid has 16 carbons including carboxyl carbon.
- Arachidonic acid has 20 carbon atoms including carboxyl carbon.
- Fatty acids could be saturated (without a double bond) or unsaturated (with one or more C=C double bonds).
- Another simple lipid is glycerol which is trihydroxy propane.
- Many lipids have both glycerol and fatty acids.
- Here the fatty acids are found esterified with glycerol.
- They can be then monoglycerides, diglycerides and triglycerides.
- These are also called fats and oils based on melting point.
- Oils have a lower melting point (e.g., gingelly oil) and hence remain as oil in winter.
- Some lipids have phosphorous and a phosphorylated organic compound in them.
- These are phospholipids.
- They are found in the cell membrane.
- Lecithin is one example.
- Some tissues especially the neural tissues have lipids with more complex structures.
Living organisms have a number of carbon compounds in which heterocyclic rings can be found.
- Some of these are nitrogen bases – adenine, guanine, cytosine, uracil, and thymine.
- When found attached to a sugar, they are called nucleosides.
- Adenosine, guanosine, thymidine, uridine, and cytidine are nucleosides.
- If a phosphate group is also found esterified in the sugar they are called nucleotides.
- Adenylic acid, thymidylic acid, guanylic acid, uridylic acid, and cytidylic acid are nucleotides.
- Nucleic acids like DNA and RNA consist of nucleotides only.
- DNA and RNA function as genetic material.
Primary and Secondary Metabolites:
In animal tissues, one notices the presence of amino acids, sugars, etc.
- These are called primary metabolites.
However, when one analysis plant, fungal and microbial cells, one would see thousands of compounds other than these called primary metabolites.
- These are called secondary metabolites.
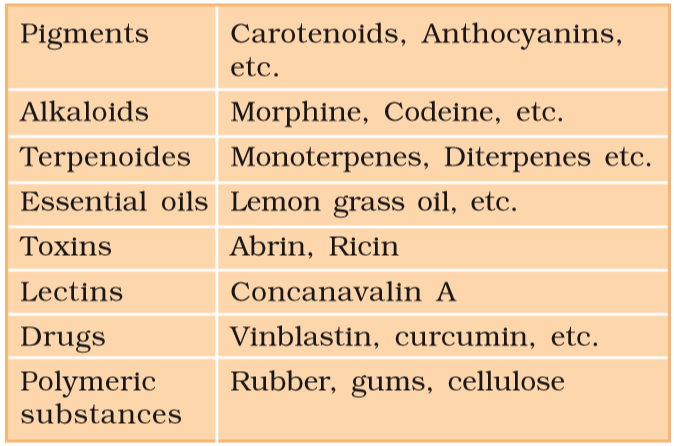
- Examples: ^^alkaloids, flavonoids, rubber, essential oils, antibiotics, colored pigments, scents, gums, and spices.^^
While primary metabolites have identifiable functions and play known roles in normal physiological processes, we do not at the moment, understand the role or functions of all the ‘secondary metabolites’ in host organisms.
- However, many of them are useful to ‘human welfare ^^(e.g., rubber, drugs, spices, scents, and pigments).^^
- Some secondary metabolites have ecological importance.
Biomacromolecules:
- There is one feature common to all those compounds found in the acid-soluble pool.
- They have molecular weights ranging from ^^18 to around 800 daltons^^ (Da) approximately.
- The acid insoluble fraction has only four types of organic compounds i.e., proteins, nucleic acids, polysaccharides, and lipids.
- These classes of compounds with the exception of lipids have molecular weights in the range of ten thousand daltons and above.
- For this very reason, biomolecules, i.e., chemical compounds found in living organisms are of two types.
- Those which have molecular weights ^^less than one thousand daltons^^ and are usually referred to as micromolecules or simply biomolecules
- Those who are found in the ^^acid insoluble fraction^^ are called macromolecules or biomacromolecules.
- The molecules in the insoluble fraction with the exception of lipids are polymeric substances.
- Lipids are indeed small molecular weight compounds and are present not only as such but also arranged into structures like cell membranes and other membranes.
- When we grind a tissue, we are disrupting the cell structure.
- Cell membranes and other membranes are broken into pieces and form vesicles that are not water-soluble.
- Therefore, these membrane fragments in the form of vesicles get separated along with the acid insoluble pool and hence in the macromolecular fraction.
- Lipids are not strictly macromolecules.
- The acid-soluble pool represents roughly the cytoplasmic composition.
- The macromolecules from the cytoplasm and organelles become the acid insoluble fraction.
- Together they represent the entire chemical composition of living tissues or organisms.
- In summary, if we represent the chemical composition of living tissue from an abundance point of view and arrange them class-wise, we observe that water is the most abundant chemical in living organisms
Proteins:
- Proteins are polypeptides.
- They are linear chains of amino acids linked by ^^peptide bonds.^^
- Each protein is a ^^polymer of amino acids.^^
- As there are 20 types of amino acids (e.g., ^^alanine, cysteine, proline, tryptophan, lysine,^^ etc.), ^^a protein is a heteropolymer and not a homopolymer.^^
- A homopolymer has only one type of monomer repeating ‘n’ a number of times.
- Dietary proteins are the source of essential amino acids.
- Therefore, amino acids can be ^^essential or non-essential.^^
- The latter are those which our body can make, while we get essential amino acids through our diet/food.
- Proteins carry out many functions in living organisms, some transport nutrients across cell membranes, some fight infectious organisms, some are hormones, some are enzymes, etc.
- ^^Collagen is the most abundant protein in the animal world and Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.^^
Polysaccharides:
- The acid-insoluble pellet also has polysaccharides (carbohydrates) as another class of macromolecules.
- ^^Polysaccharides are long chains of sugars.^^
- They are threads (literally cotton threads) containing different monosaccharides as building blocks.
- For example, cellulose is a polymeric polysaccharide consisting of only one type of monosaccharide i.e., glucose.
- ^^Cellulose is a homopolymer.^^
- Starch is a variant of this but is present as a storehouse of energy in plant tissues.
- Animals have another variant called glycogen.
- Inulin is a polymer of fructose.
- In a polysaccharide chain (say glycogen), the right end is called the reducing end, and the left end is called the non-reducing end.
- It has branches as shown in the form of a cartoon.
- Starch forms helical secondary structures.
- ^^In fact, starch can hold I2 molecules in the helical portion.^^
- ^^The starch-I2 is blue in color.^^
- Cellulose does not contain complex helices and ^^hence cannot hold I2.^^
- Plant cell walls are made of cellulose.
- ^^Paper made from plant pulp and cotton fiber is cellulosic.^^
- There are more complex polysaccharides in nature.
- They have as building blocks, amino sugars and chemically modified sugars (e.g., glucosamine, N-acetyl galactosamine, etc.).
- Exoskeletons of arthropods, for example, have a complex polysaccharide called chitin.
- ^^These complex polysaccharides are mostly homopolymers.^^
Nucleic Acid:
- The other type of macromolecule that one would find in ^^the acid-insoluble fraction^^ of any living tissue is a nucleic acid.
- These are polynucleotides.
- Together with polysaccharides and polypeptides, these comprise the true macromolecular fraction of any living tissue or cell.
- ^^For nucleic acids, the building block is a nucleotide.^^
- A nucleotide has three chemically distinct components.
- One is a heterocyclic compound
- the second is a ^^monosaccharide^^
- the third is ^^phosphoric acid or phosphate.^^
- The heterocyclic compounds in nucleic acids are the nitrogenous bases named ^^adenine, guanine, uracil, cytosine, and thymine.^^
- ^^Adenine and Guanine have substituted purines while the rest are substituted pyrimidines.^^
- The skeletal heterocyclic ring is called purine and pyrimidine respectively.
- The sugar found in polynucleotides is either ^^ribose (a monosaccharide pentose) or 2’ deoxyribose.^^
- A nucleic acid containing deoxyribose is called ^^deoxyribonucleic acid (DNA) while that which contains ribose is called ribonucleic acid (RNA).^^
Structure of Proteins:
- Proteins are heteropolymers containing strings of amino acids.
- The structure of molecules means different things in different contexts.
- In inorganic chemistry, the structure invariably refers to the molecular formulae (^^e.g., NaCl, MgCl2, etc^^.)
- Organic chemists always write a two-dimensional view of the molecules while representing the structure of the molecules (^^e.g., benzene, naphthalene, etc.^^).
- Physicists conjure up the three-dimensional views of molecular structures while biologists describe the protein structure at four levels.
- The sequence of amino acids i.e., the positional information in a protein – which is the first amino acid, which is the second, and so on – is called the primary structure of a protein.
- A protein is imagined as a line, the left end represented by the first amino acid and the right end represented by the last amino acid.
- The first amino acid is also called an N-terminal amino acid.
- The last amino acid is called the C-terminal amino acid.
- A protein thread does not exist throughout as an extended rigid rod.
- The thread is folded in the form of a helix (similar to a revolving staircase).
- Of course, only some portions of the protein thread are arranged in the form of a helix. In proteins, only right-handed helices are observed.
- Other regions of the protein thread are folded into other forms in what is called the secondary structure.
- In addition, the long protein chain is also folded upon itself like a hollow woolen ball, giving rise to the tertiary structure.
- This gives us a 3- dimensional view of a protein.
- Tertiary structure is absolutely necessary for the many biological activities of proteins.
- Some proteins are an assembly of more than one polypeptide or subunit.
- The manner in which these individual folded polypeptides or subunits are arranged with respect to each other (e.g. linear string of spheres, spheres arranged one upon each other in the form of a cube or plate, etc.) is the architecture of a protein otherwise called the quaternary structure of a protein.
- Adult human hemoglobin consists of 4 subunits.
- Two of these are identical to each other.
- Hence, two subunits of α type and two subunits of β type together constitute the human hemoglobin (Hb).
Nature of Bond Linking Monomers in a Polymer:
- In a polypeptide or a protein, amino acids are linked by a peptide bond which is formed when the carboxyl (-COOH) group of one amino acid reacts with the amino (-NH2 ) group of the next amino acid with the elimination of a water moiety (the process is called dehydration).
- In a polysaccharide, the individual monosaccharides are linked by a glycosidic bond.
- This bond is also formed by dehydration.
- This bond is formed between two carbon atoms of two adjacent monosaccharides.
- In a nucleic acid, a phosphate moiety links the 3’-carbon of one sugar of one nucleotide to the 5’-carbon of the sugar of the succeeding nucleotide.
- The bond between the phosphate and hydroxyl group of sugar is an ester bond.
- As there is one such ester bond on either side, it is called a phosphodiester bond.
- Nucleic acids exhibit a wide variety of secondary structures.
- For example, one of the secondary structures exhibited by DNA is the famous Watson-Crick model.
- This model says that DNA exists as a double helix.
- The two strands of polynucleotides are antiparallel i.e., run in the opposite direction.
- The backbone is formed by the sugar-phosphate-sugar chain.
- The nitrogen bases are projected more or less perpendicular to this backbone but face inside.
- A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand.
- There are two hydrogen bonds between A and T and three hydrogen bonds between G and C.
- Each strand appears like a helical staircase.
- Each step of the ascent is represented by a pair of bases.
- At each step of ascent, the strand turns 36°.
- One full turn of the helical strand would involve ten steps or ten base pairs.
- The pitch would be 34Å.
- The rise per base pair would be 3.4Å.
- This form of DNA is called BDNA.
Dynamic State of Body Constituents - Concept of Metabolism:
- One of the greatest discoveries ever made was the observation that all these biomolecules have a turnover.
- This means that they are constantly being changed into some other biomolecules and also made from some other biomolecules.
- This breaking and making are through chemical reactions constantly occurring in living organisms.
- Together all these chemical reactions are called metabolism.
- Each of the metabolic reactions results in the transformation of biomolecules.
- A few examples of such metabolic transformations are:
- the removal of CO2 from amino acids making an amino acid into an amine
- the removal of an amino group in a nucleotide base
- the hydrolysis of a glycosidic bond in a disaccharide, etc.
- The majority of these metabolic reactions do not occur in isolation but are always linked to some other reactions.
- In other words, metabolites are converted into each other in a series of linked reactions called metabolic pathways.
- These pathways are either linear or circular.
- These pathways crisscross each other, i.e., there are traffic junctions.
- The flow of metabolites through the metabolic pathway has a definite rate and direction like automobile traffic.
- This metabolite flow is called the dynamic state of body constituents.
- Another feature of these metabolic reactions is that every chemical reaction is a catalyzed reaction.
- There is no uncatalyzed metabolic conversion in living systems.
- Even CO2 dissolving in water, a physical process, is a catalyzed reaction in living systems.
- The catalysts which hasten the rate of a given metabolic conversation are also proteins.
- These proteins with catalytic power are named enzymes.
Metabolic Basis of Living:
- Metabolic pathways can lead to a more complex structure from a simpler structure (for example, acetic acid becomes cholesterol) or lead to a simpler structure from a complex structure (for example, glucose becomes lactic acid in our skeletal muscle).
- The former cases are called biosynthetic pathways or anabolic pathways.
- The latter constitute degradation and hence are called catabolic pathways.
- Anabolic pathways, as expected, consume energy.
- Assembly of a protein from amino acids requires energy input.
- On the other hand, catabolic pathways lead to the release of energy.
- For example, when glucose is degraded to lactic acid in our skeletal muscle, energy is liberated.
- This metabolic pathway from glucose to lactic acid which occurs in 10 metabolic steps is called glycolysis.
- Living organisms have learned to trap this energy liberated during degradation and store it in the form of chemical bonds.
- As and when needed, this bond energy is utilized for biosynthetic, osmotic, and mechanical work that we perform.
- The most important form of energy currency in living systems is the bond energy in a chemical called ^^adenosine triphosphate (ATP).^^
The Living State:
- The most important fact of biological systems is that all living organisms exist in a steady-state characterized by concentrations of each of these biomolecules.
- These biomolecules are in metabolic flux.
- Any chemical or physical process moves spontaneously to equilibrium.
- The steady state is a non-equilibrium state.
- One should remember from physics that systems at equilibrium cannot perform work.
- As living organisms work continuously, they cannot afford to reach equilibrium.
- Hence the living state is a non-equilibrium steady state to be able to perform work; the living process is a constant effort to prevent falling into equilibrium.
- This is achieved by energy input.
- Metabolism provides a mechanism for the production of energy.
- Hence the living state and metabolism are synonymous.
- Without metabolism, there cannot be a living state