Liquids and Solids: Kinetic Molecular Model and Intermolecular Forces
1/29
Earn XP
Description and Tags
General Chemistry II
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Kinetic Molecular Model
describes matter as made up of tiny particles in constant motion
explains the physical properties of matter, particularly gases, in terms of the motion and behavior of tiny particles
Particles are in constant, random motion, and their collisions and energy levels determine macroscopic properties like temperature and pressure.
Kinetic Molecular Model: SOLIDS
solid particles are closely packed in a fixed, ordered structure and experience strong intermolecular forces, causing them to vibrate in place but not move around freely.
solids have a definite shape and volume due to these strong forces and limited particle movement.
Kinetic Molecular Model: LIQUIDS
particles are in constant, random motion, and their kinetic energy is related to the liquid’s temperature.
the intermolecular forces are strong enough to keep the particles close together, but not so strong as to fix them in a specific position, allowing them to move past each other and giving liquids their fluidity
Characteristic Properties of Gases, Liquids, and Solids
Intermolecular Forces
the attractive or repulsive forces that exist between molecules, influencing their physical properties
They are weaker than intramolecular forces (like covalent bounds) that holds atoms together within a molecule.
London Dispersion Forces
also known as dispersion forces or instantaneous dipole-induced dipole force
type of intermolecular force - that is, a force that occurs between molecules
the weakest of all the van der Waals forces, but they are present in all molecules, whether polar or nonpolar
What Causes London Dispersion Forces?
Electrons are constantly moving around the nucleus of atoms and molecules
At any instant, the distribution of these electrons might be uneven - this creates a temporary (instantaneous) dipole.
This temporary dipole can influence nearby atoms or molecules, causing them to also form induced dipoles.
These two temporary dipoles can then attract each other, and that attraction is the London dispersion force.
Key Characteristics
Weak, but universal: Even noble gases like helium and argon, and nonpolar molecules like O2 OR ch4, exhibit London dispersion forces.
Strength increases with:
Molecular size/mass: Larger atoms/molecule have more electrons, so their electron cloud is more polarizable, which increases dispersion force
Surface area: Long, straight molecules have more contact area and thus more opportunity for dispersion interactions compared to compact or branched molecules.
Dipole-Dipole Forces
attractive force that occur between the positive end of one polar molecule and the negative end of another polar molecule
exist only in polar-molecules - that is, molecules that have a permanent dipole
How Are Dipole-Dipole Forces Formed?
Molecules have polar bonds due to differences in electronegativity
These bonds cause a separation of charge, making one end of the molecule partially positive (symbol) and the other partially negative (symbol)
In a group of such polar molecules, the positive end of one molecule is electrostatically attracted to the negative end of a neighboring molecule
This attraction between opposite charges is the dipole-dipole force.
Ion-Dipole Forces
Attractive forces that occur between a fully charged ion (either a cation or anion) and the partially charged end of a polar molecule (dipole).
How do Ion-Dipole Forces form?
An ion( from an iconic compound like NA + or CI -) is present in a polar substance (usually a liquid like water).
Water molecules (or any polar molecules) have partial positive and partial negative changes due to uneven sharing of electrons.
The ion is attracted to the oppositely charged side of the polar molecule
1. Cations (eg -, NA +) are attracted to the negative (symbol) side of the dipole (like oxygen in H20).
2. Anions (e.g., CI -) are attracted to the positive (symbol) side (like hydrogen in H20)
The interaction is called an ion-dipole force.
Strength of Ion-Dipole Forces
Stronger than dipole-dipole and dispersion forces because:
Ions carry full charges (+1, -1, etc.)
Dipoles only have partial charges (symbol)
More polar solvents (like water) lead to stronger ion-dipole forces.
Hydrogen Bonding
A strong dipole-dipole attraction that occurs when a hydrogen atom, covalently bonded to a highly electronegative atom ( such as nitrogen (N), oxygen (O), or fluorine (F)), is attracted to another electronegative atom with a lone pair of electrons.
Conditions for Hydrogen Bonding
Hydrogen bonding can only occur when three conditions are met:
Hydrogen (H) is directly bonded to N, O, or F.
The N, O, or F atom is small and highly electronegative, which makes the H-X bond very polar.
There is a lone pair of electrons on another nearby N, O, or F atom that attracts the hydrogen.
How Does it Form?
Let’s look at water (H20) as an example: Each water molecule has two polar O-H bonds. Oxygen is more electronegative, so it pulls electron density toward itself, making
H -> partially positive (symbol)
O -> partially negative (symbol)
The hydrogen (symbol) of one water molecule is attracted to the oxygen (symbol) of another
Properties of Liquids
Liquids ->
Liquids
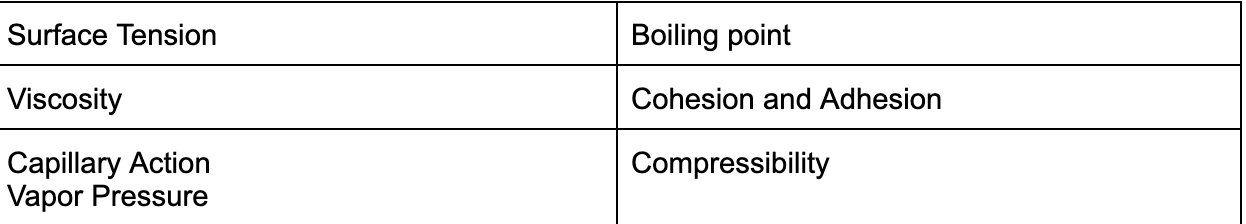
Solids
Particles in solids are not moving in the same manner as those in liquids or gases
Particles simply vibrate and rotate in place rather than move about
Solids generally held together by iconic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong
Solids are classified as:
Amorphous solids
Crystalline solids
Amorphous SOLID
Do not have much order in their structures
An amorphous solid does not possess a well-defined arrangement and long-range molecular order.
An amorphous solid Melts Over a Ranger. They soften and melt over a range of temperatures rather than at a specific point.
Irregular Fracture When broken they fracture coincidentally
Regular Edgers and forces
When broken
Crystalline SOLID
hat make up the solid exist in a regular, well-defined arrangement
A crystalline solid possesses rigid and long-range order. In a crystalline solid, atoms, molecules or ions occupy specific (predictable) positions
A crystalline solid have Sharp melting point. They melt at a specific, defined temperature
Isotropic:
Their properties are generally the same in all directions.
Anisotropic.
Their properties can vary depending on the direction of measurement due to the ordered structure
Crystalline solids
Processes rigid and long-range order
Unit cell is the basic repeating structural unit
Lattice is an ordered array of points describing the arrangement of particles that forn a cyrstal
Types of crystals:
Ionic crystals
Molecular
Covalent
Metalic
At lattice points:
Atoms
Molecules
Ions
Ionic crystals
Lattice points occupied by cations and anions
Held together by electrostatic attraction
Hard, brittle, high melting point
Poor conductor of heat and electricity
Covalent crystals
Lattice points occupied by atoms
Held together by covalent bonds
Hard, high melting point
Poor conductor of heat and electricity
Molecular crystals
Lattice points occupied by molecules
Held together by intermolecular forces
Soft, low melting point
Poor conductor of heat and electricity
Metallic crystals
Lattice points occupied by metal atoms
Held together by metallic bonds
Soft to hard, low to high melting point
Good conductors of heat and electricity