Matter and its Properties
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
Anything that has mass and takes up space (volume)
matter

3 States of Matter
-Solid, Liquid, and Gas
Have a definite shape and volume.
-Solids

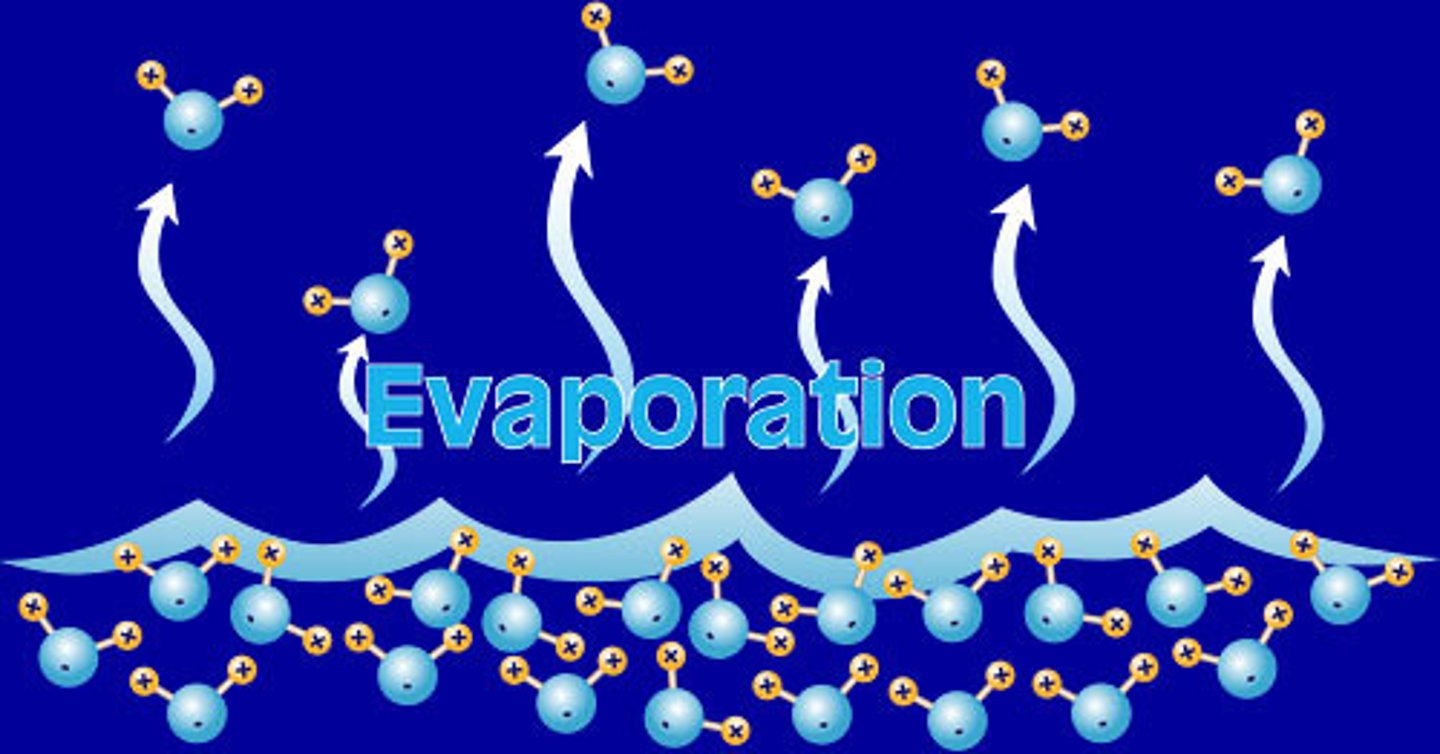
When a liquid changes to a gas.
evaporation
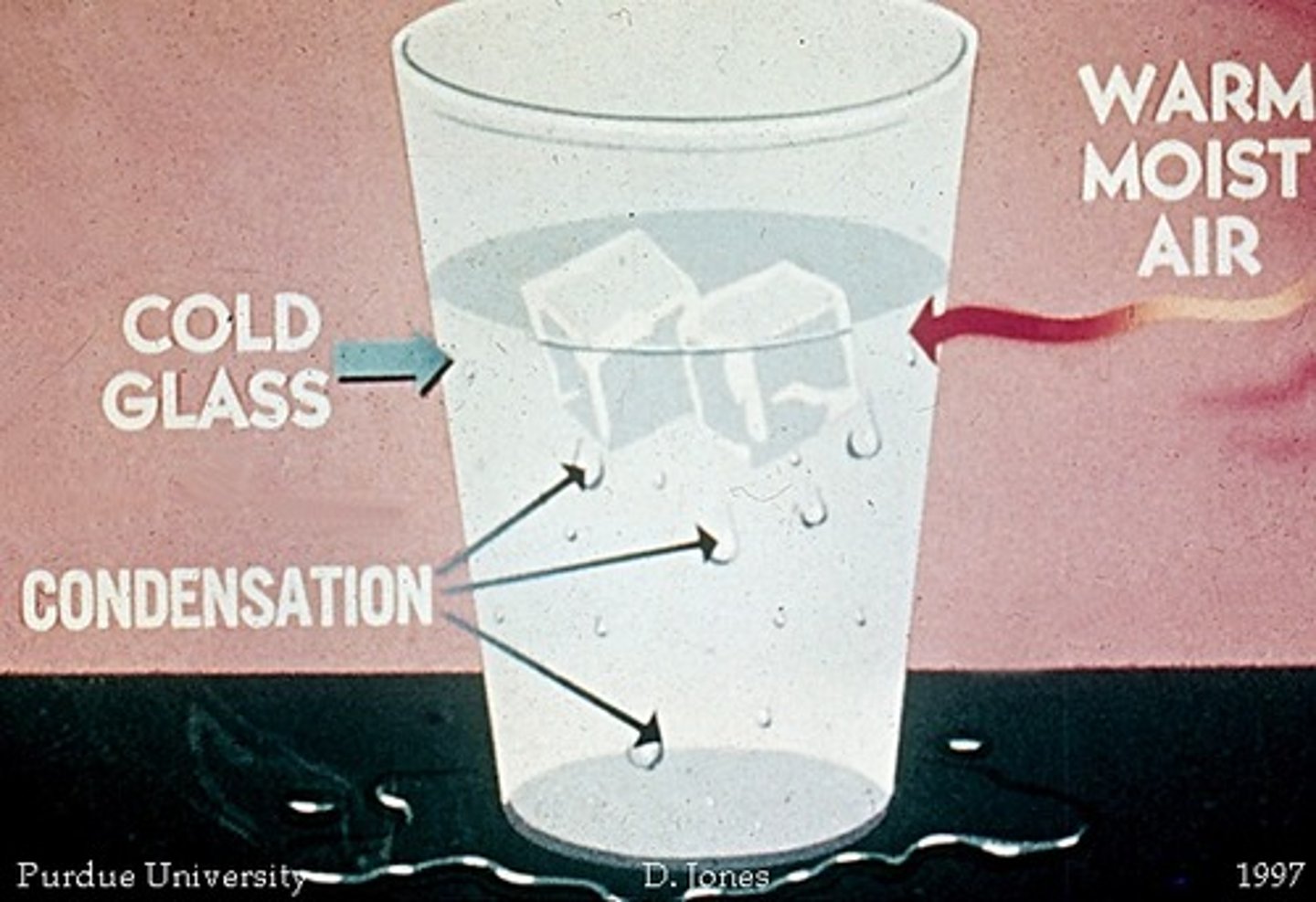
Formation of liquid drops of water from water vapor.
condensation
Observable characteristics that make objects different from each other.
Ex. Pencil is wooden
Ex. Cotton Ball is soft
Physical properties

Tiny pieces that make up the materials in matter. They are too small to be seen without a microscope
particles

Change from liquid to a solid
freezing
A measure of the amount of space occupied by matter
volume

A measure of the amount of matter.
mass
distance between two points.
length

What happens when matter is heated?
If matter is heated, it will expand.
The molecules will speed up and spread out.

resting on the surface of a liquid.
floating

What are three properties of a paper clip?
Silver, Curved, Shiny

Name some properties of water
-flows downhill
-colorless
-odoress
-75% of the earth
What DISSOLVES in water?
-Sugar
-Salt
-Powdered drinks

What does NOT dissolve in water?
-oil
-sand
-rocks

What happens to object with a density less than water.
They float

Have no definite shape or volume. Fill their container.
gases

Take the shape of their container. They have a definite volume.
liquids

How to calculate the volume of a rectangular prism
length x width x height

The ability of a substance to be dissolved.
solubility

The measure of the pull of gravity on an object.
weight

What is the freezing point of water?
32 degrees Fahrenheit
0 degrees Celsius

What is the boiling point of water?
212 degrees Fahrenheit
100 degrees Celsius

What is the Law of Conservation of Matter?
Matter can not be created or destroyed. It only changes form.

A combination of two kinds of matter in which each keeps its own properties and can be separated.
mixture

gas
A state of matter with no definite shape or volume

solid
Definite shape and volume

rust
the formation of reddish-brown ferric oxides on iron by low-temperature oxidation in the presence of water
water
H2O
volume
The amount of space an object takes up
rheyn
ibz
somalarah
lemyi
proleo
daily
tresib
nocil
trappatr
xile
alha
inalan
aras
cargia
sia
dodaleg
nasoj
melon
tama
rovtic
physical change
A change in a substance that does not involve a change in the identity of the substance
chemical change
A change in matter that produces one or more new substances
ertniw
winter
Walter Payton
Football
Chicago Bears
Running Back
personification
the giving of human qualities to an animal, object, or idea
The clouds were balloons floating across the sky.
metaphor
Jude is as fast as lightning.
simile
six of one, half a dozen of the other
idiom - Said when neither of two choices is better than the other
3 x 3
9
6 x 8
48
13 x 12
156
7 x 8
56
buoyancy
the ability or tendency to float in water or air or some other fluid.
density
the degree of compactness of a substance.
fluid
any substance that can flow
architect
a person who designs buildings
food web
a community of organisms where there are several interrelated food chains
predator
animal that hunts other animals for food
prey
animal hunted or caught for food
decomposer
an organism that breaks down wastes and dead organisms
producer
an organism that can make its own food.
abiotic
non-living
biotic
living
graduated cylinder
instrument used to measure volume of a liquid
mass
the amount of matter in an object
periodic table of the elements
a table that classifies elements by their physical and chemical properties; rows are called periods; columns are called groups
element #1 on the Periodic Table of Elements
hydrogen
erolfel
belak
snave
nniuq
orevol
wehttam
irrational
not based on reason or logic
irresponsible
not reliable; untrustworthy; not answering for one's actions
intercept
to stop something from reaching its intended destination
intersection
the point at which two lines or roads meet and cut across each other
irregular
not following a pattern; not regular
illogical
not done according to reason
intercede
mediate
interaction
the effect of one factor (such as environment) depends on another factor (such as heredity)