Lecture 36 - Hypothalamic-Pituitary-Adrenal Axis Disorders
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
what are the adrenal glands
located at top of each kidney
adrenal medulla (10% of gland) → secretes catecholamines, stimulus for secretion of sympathetic nervous system
adrenal cortex (90% of gland) → secretes mineralocorticoids/glucocorticoids (corticosteroids), androgens
mineralocorticoids e.g. aldosterone = promote sodium retention in renal tubules and potassium excretion
glucocorticoids e.g. cortisol = mediate stress response and homeostasis
androgens e.g. testosterone and estradiol
what is cholesterol
secreted from cortex of adrenal gland → precursor to steroid hormones
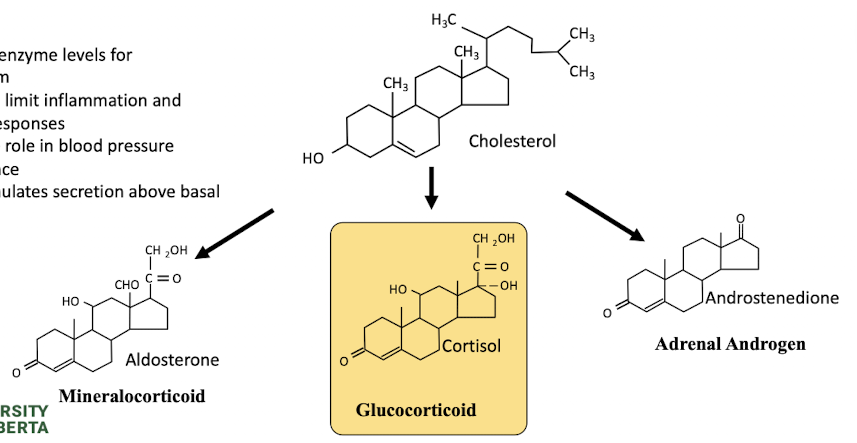
what is cortisol
main hormone of the HPA axis
regulate enzyme levels for metabolism
may act to limit inflammation and immune responses, role in BP maintenance
stress stimulates secretion above basal levels
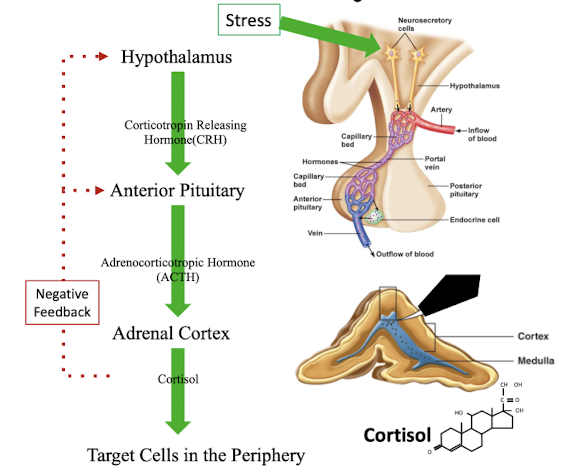
what are the steps of the activation of the hypothalamic pituitary adrenal axis
stress → act on hypothalamus → release of corticotropin releasing hormone (CRH) → anterior pituitary releases adrenocorticotropic releasing hormone (ACTH) → act on adrenal cortex to stimulate cortisol release → target cells in periphery
cortisol negative feedback loop to inhibit release of CRH and ACTH
how can adrenal disorders be categorized
hypperfunction or hypofunction of the adrenal gland
can have significant clinical functions → hormones help regulate BP, metabolism, immune response, stress response, bodily functions
what are types of hyperfunction of the adrenal glands
Cushing’s Syndrome (hypercortisolism), hyperaldosteronism
what are types of hypofunction of the adrenal glands
Addison’s disease (adrenal insufficiency), genetic abnormalities of steroidogenesis
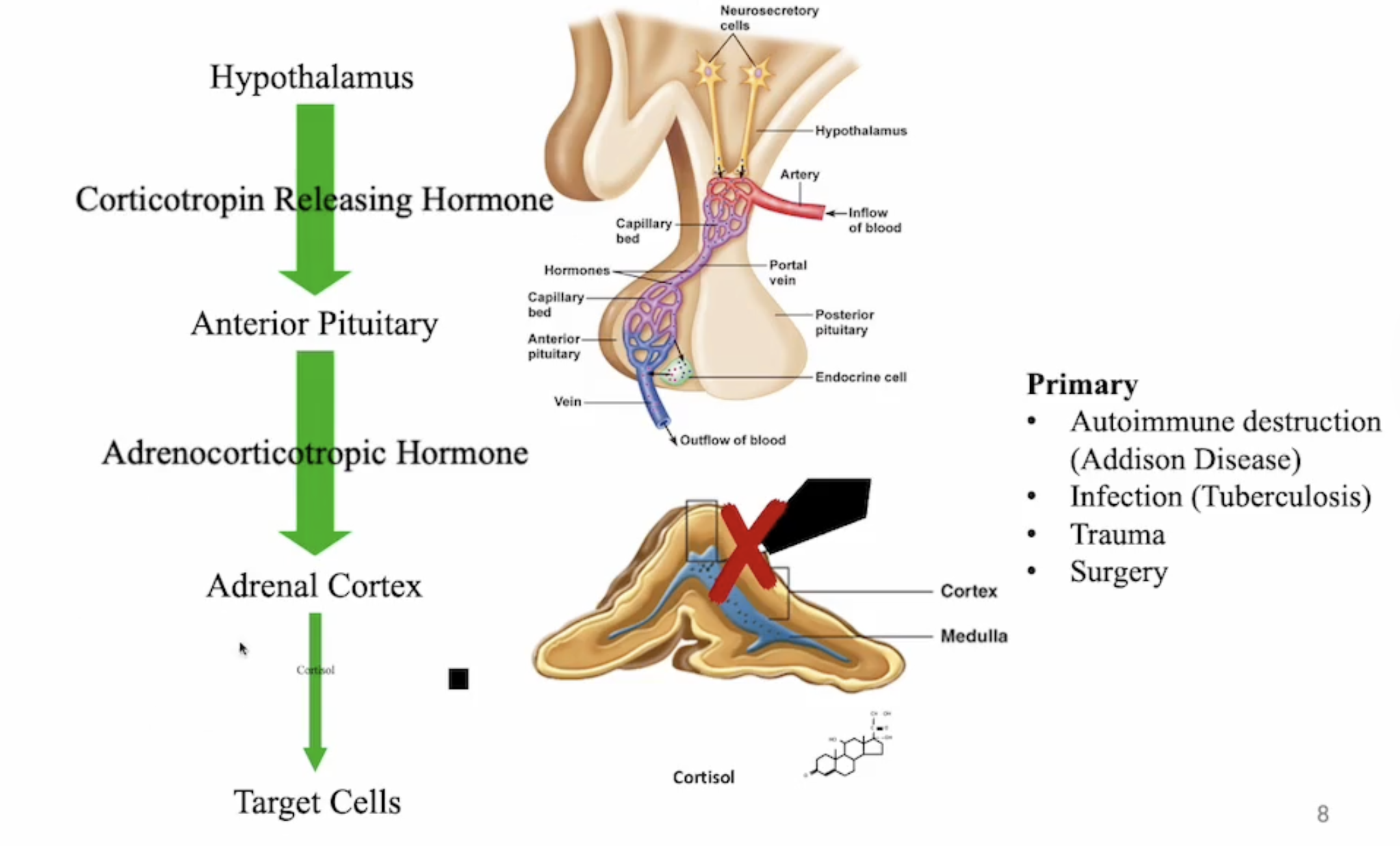
what is primary adrenocortical insufficiency
destruction or dysfunction of adrenal cortex (Addison’s disease, infection e.g. tuberculosis, trauma, surgery)
usually caused by autoimmune dysfunction
more common in women
results in deficiencies in cortisol, aldosterone, androgens
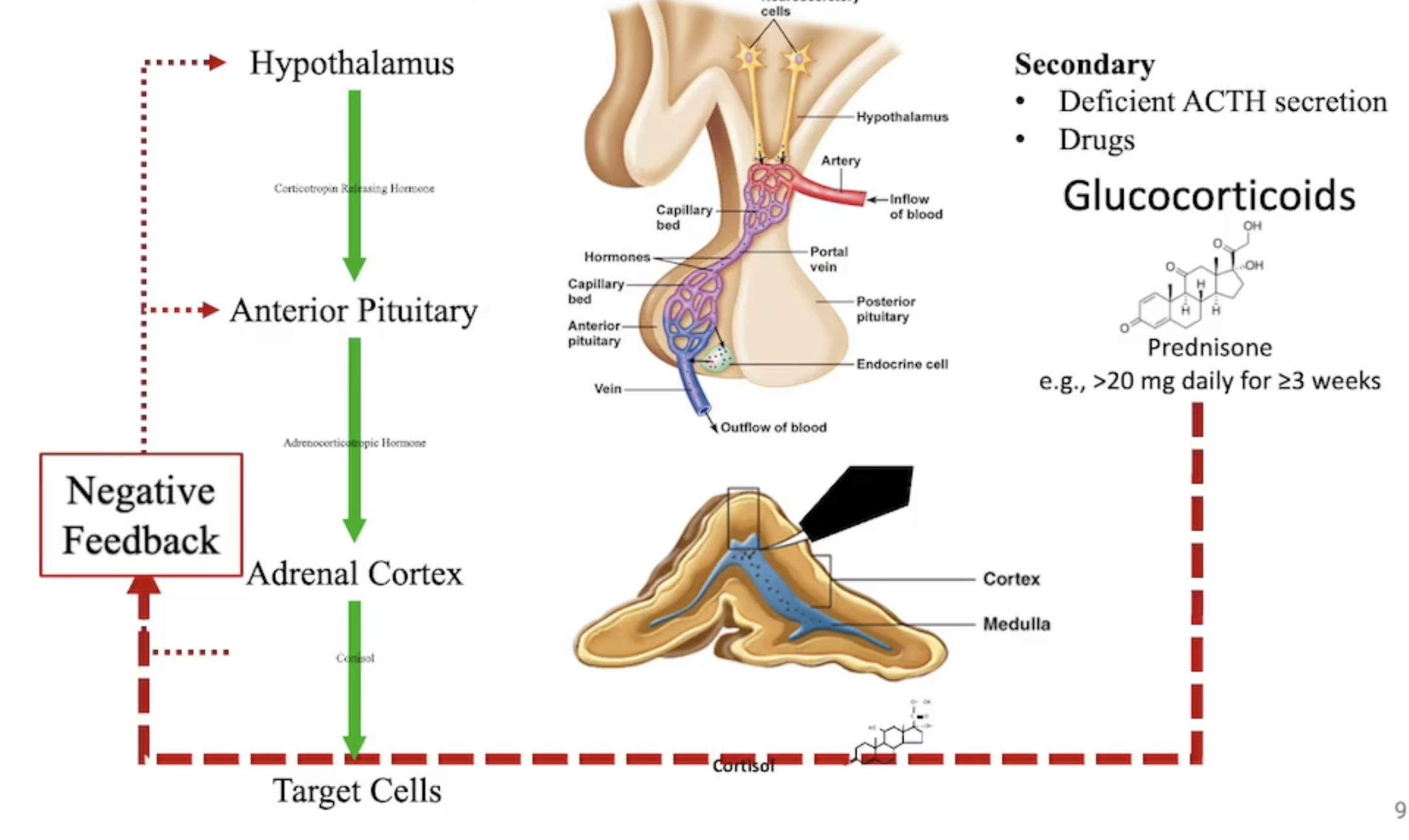
what is secondary adrenocortical insufficiency
deficient secretion of adrenocorticotropic hormone (ACTH)
glucocorticoid therapy - important implication if therapy is stopped or dose reduced
what is the clinical presentation of adrenocortical insufficiency
weakness, lethargy, fatigue
anorexia, weight loss
abdominal symptoms - nausea, vomiting, abdominal pain
hypoglycemia
hyperpigmentation of skin (only in primary insufficiency from excessive ACTH)
in primary: mineralocorticoid deficiency - hypotension, dehydration, hyponatremia, hyperkalemia
what are lab tests to diagnose adrenocortical insufficiency
morning plasma cortisol (6am - 9am) <80 nmol/L (ref 138-635 nmol/L)
ACTH will be high in primary insufficiency, low in secondary insufficiency
corticotropin (or cosyndropin) stimulation test
measure plasma cortisol at baseline
administer 250 ug synthetic ACTH
repeat plasma cortisol in 30-60min → > 500 nmol/L rules out primary adrenal insufficiency (adrenal cortex still works)
what are drug-test interactions for adrenocortical insufficiency
oral contraceptives → increase cortisol-binding globulin (CBG) = elevated total cortisol levels
what is the appropriate dosing regimen for primary adrenocortical insufficiency
hydrocortisone 15-25 mg/day
2/3 dose in the morning, 1/3 dose late in the afternoon → mimic normal endogenous release
mineralocorticoid → fludrocortisone 0.05-0.1mg daily
what is the consideration of stress dosing in primary adrenocortical insufficiency
patient is unable to mount a normal cortisol response (increase in BP and blood glucose) to stressful events → leading cause of adrenal crises
to prevent: increase dose of glucocorticoid during major illness or injury
increase is individualized based on patient and stressor
dose is generally doubled during febrile illness
continue with increased dose until feeling better
injectable hydrocortisone may be required in diarrhea/vomiting
if patient using mineralocorticoid - should increase 2-3x regular dose for hot weather, strenuous exercise (sweating and fluid loss) → watch for fluid retention and HTN
what is secondary adrenocortical insufficiency management
patients may need glucocorticoid replacement, but not mineralocorticoid replacement
adrenal cortex not damaged
aldosterone production and release managed by RAAS
dose of corticosteroid depends on symptoms, ACTH levels, and response to corticotropin stimulation test
most patients require stress dose steroids
counselling on tapered withdrawal of corticosteroids important (abrupt withdrawal can cause adrenal crisis)
what is Cushing’s syndrome
clinical condition from chronic, excessive levels of endogenous cortisol or exogenous corticosteroids
most common cause: drug related (prolonged high dose/potency glucocorticoids)
other causes: adrenal adenomas, adrenal carcinomas, ectopic ACTH secretion (neuroendocrine tumours)
what is Cushing’s disease
excessive cortisol levels caused by overproduction and release of ACTH from a pituitary tumour
what is the clinical presentation of adrenal excess
fatigue and weakness
increased body weight, redistribution of body fat (centripetal obesity, facial rounding, thin extremeties)
hypertension
hirsutism
amenorrhea
muscle wasting
thinning of skin
easy bruising
what are lab tests to establish hypercortisolism
measure level of cortisol → 24h urine collection or midnight plasma or salivary cortisol to see if dinural pattern is intact
dexamethasone suppression test
1mg administered orally at bedtime
morning cortisol measured next day: <50nmol/L rules out Cushing syndrome (body responding appropriately), >50nmol/L confirms cushing syndrome
neither test identifies etiology of hypercortisolism
false positive: exogenous glucocorticoid administration, acute illness, pregnancy, oral contraceptive use
what is the management of Cushing disease
surgery or pituitary radiation to reduce size of pituitary tumor
goal: normalize cortisol levels and alleviate symptoms, help with preoperative management or as adjunctive therapy to surgery/radiation if symptoms persist
what are pharmacologic options for managing Cushing disease
enzyme inhibitors to decrease cortisol synthesis:
ketoconazole (monitor LFTs)
mitotane (can block thyroxine synthesis)
modulate ACTH secretion from pituitary
carbergoline (dopamine agonist)
pasireotide (somatostatin analogue)
what is the management of Cushing syndrome
re-evaluate the indication for corticosteroid ongoing use
warning: continuously monitor for symptoms of adrenal insufficiency if patient using exogenous corticosteroids for prolonged periods of time
e.g. ≥20mg prednisone for ≥3 weeks
if using steroids for < 3 weeks or is administering on alternate day therapy = less likely to develop
taper dose to avoid adrenal insufficiency
what is hyperprolactinemia
state of persistently elevated prolactin levels
secretion is regulated primarily by tonic inhibition from dopamine
what are clinical manifestations of hyperprolactinemia
galactorrhea
hypogonadism → irregular menses, sexual dysfunction, infertility, osteoporosis
what are the causes of hyperprolactinemia
usually affects women of reproductive age
most commonly caused by prolactinomas (benign prolactin-secreting pituitary tumor)
drugs that antagonize dopamine:
antipsychotic medications (dopamine blockade)
antidepressants (elevated serotonin = stimulates prolactin secretion)
metoclopramide and domperidone (dopamine antagonists)
less common: elevated TRH (hypothyroidism) or estrogen stimulating lactotrophs (cells that secrete prolactin from anterior pituitary)
what is the management of hyperprolactinemia
stop medications that antagonize dopamine
use dopamine agonists for prolactinomas → cabergoline, bromocriptine
monitor for effectiveness by following prolactin levels