4. Phenolic compounds
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
79 Terms
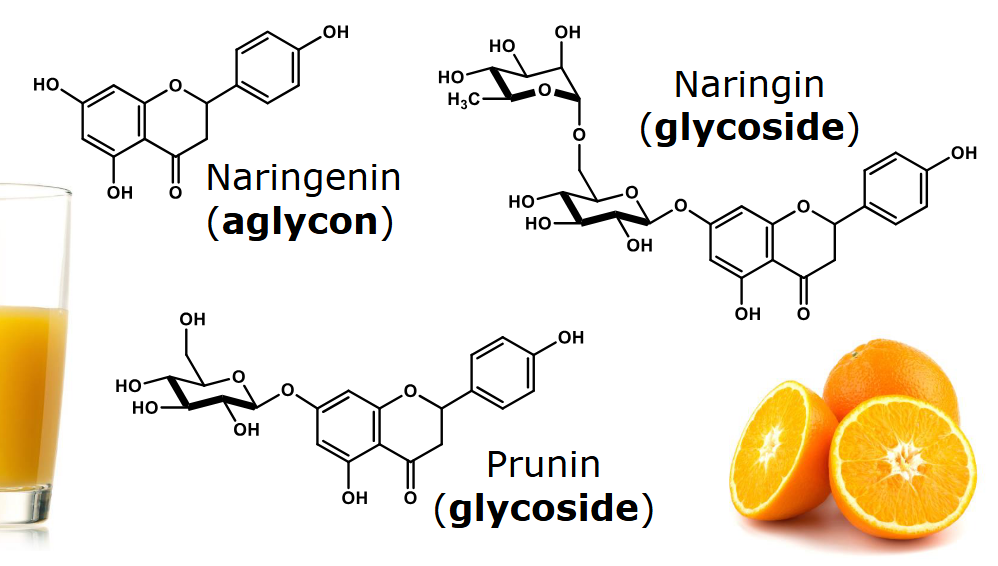
Example of phenolic as glycoside in plants
Naringenin has no sugars attached
Prunin has one sugar attached
Naringin has two sugars attached
Phenolic compounds in food
They are minor constituents of food
They have no noteworthy nutritional value
Present in plant products
Most important sources; fruits, beverages and vegetables
Has an influence on food properties.
Definition of phenolic compounds
A molecule that contains at least one aromatic ring with one or more hydroxyl groups
Examples of phenolic rich foods
Coffee
Tea
Chocolate
Berries and grapes
And products that are made of these: wine and juices
Herbs and spices (but are consumed in small amounts)
Can contain 10-30% phenolics on dry matter basis
Influence of phenolics on color & appearance
Gives berries blue, purple & red color
Responsible for browning upon phenolic oxidation
Can cause turbidity in beer
Influence of phenolics on flavor
Aroma formation reactions (e.g. during roasting of coffee beans)
Volatile phenolics as odors (e.g. vanilla)
Gives bitter taste in grapefruit
Astringency (dry feel in mouth when drinking wine)
Influence of phenolics on stability & shelf-life
Antioxidant activity
Antimicrobial properties
Structural variations in phenolics
Different groups create different properties
Three important parameters:
Polarity
Reactivity
Size of the conjugated system
Polarity, reactivity and size of conjugated system in phenolics
Polarity: Phenolics are typically medium polar → limited water solubility
Reactivity: Reactive phenolics often also possess high antioxidant activity
Size of conjugated system: Conjugated system < 8 bonds = no color
Effect of hydroxylation on phenolics
Increases water solubility & increases reactivity
O-diphenol moiety is formed
Highly relevant for oxidation reactions and antioxidant activity
Effect of methylation on phenolics
Decreases water solubility & decreases reactivity
Binds to one of aromatic OH groups
Therefore water solubility is reduced as phenolic is less soluble
Effect of glycosylation on phenolics
Increases water solubility & decreases reactivity
The attachment of a glycosidic group to an aromatic OH group
Effect of carboxylation on phenolics
Increases water solubility & decreases pKa
Attaches to aromatic ring
May lower food pH
Effect of extending conjugated system on phenolics
Increases reactivity & increases light absorbance
By attaching (for example) alkynyl groups to the aromatic ring
Longer = possibly color
Effect of formation of fused ring system on phenolics
Possible extension of conjugated system
Structure of o-diphenol & relevance
Highly relevant for oxidation reactions and antioxidant activity
Core structures of monomeric phenolics
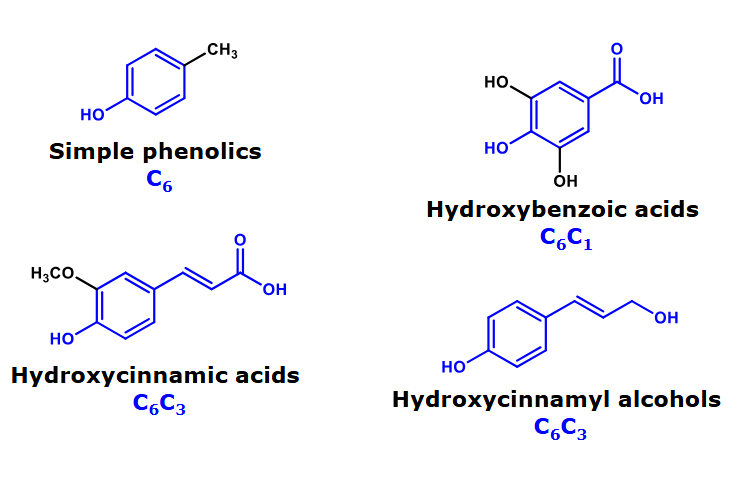
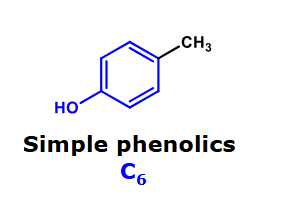
Simple phenolics
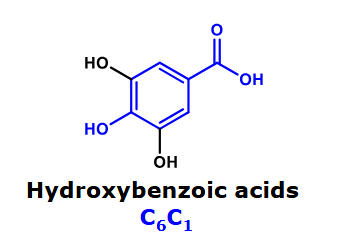
Hydroxybenzoic acids
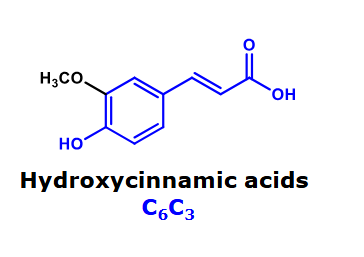
Hydroxycinnamic acids
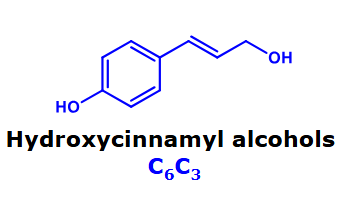
Hydroxycinnamyl alcohols
Relevance of hydroxycinnamic acids
Hydroxycinnamic acids & derivatives are present in grains and coffee beans.
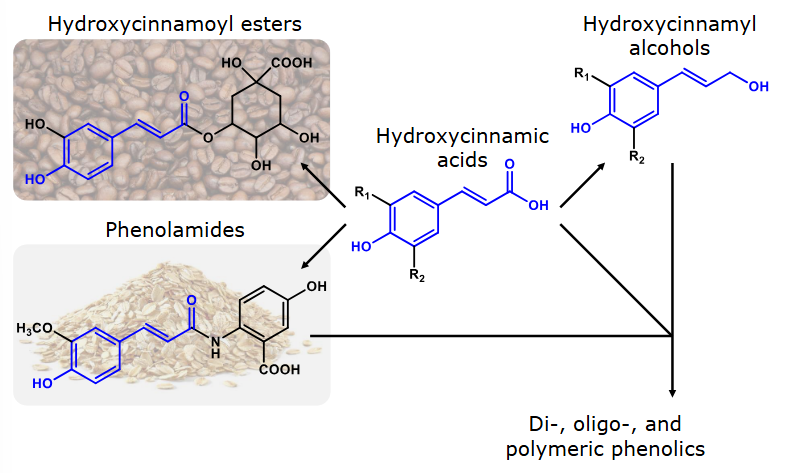
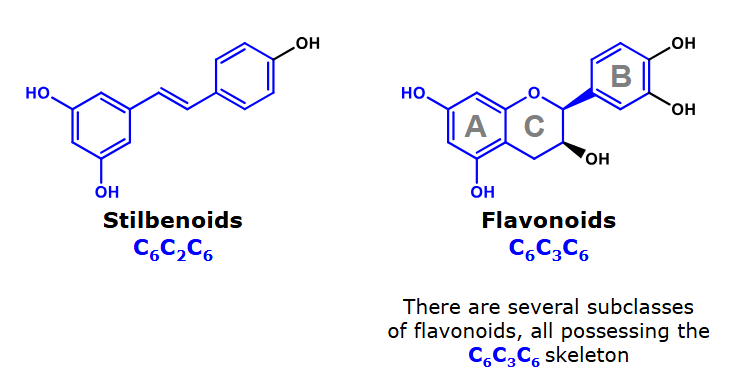
Two core structures of monomeric phenolics
Stillbenoids are found in grapes and wines.
Four important classes of di, oligo and polymeric phenolics
Lignans
Lignins
Condensed tannins
Hydrolysable tannins
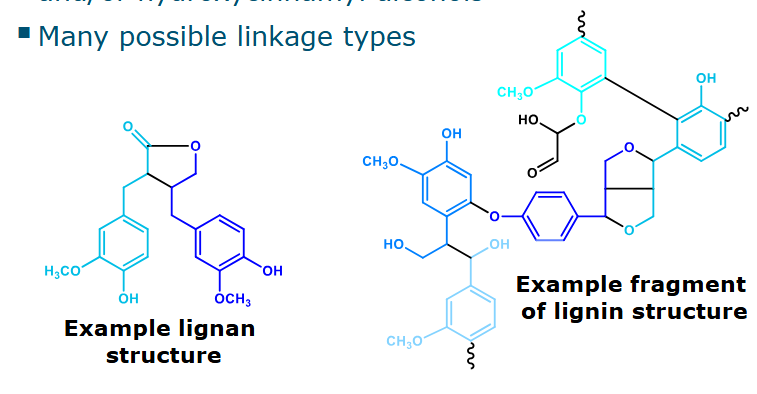
Lignans & lignins
Monomeric building blocks: hydroxycinnamic acids and/or hydroxycinnamyl alcohols
Many possible linkage types
Lignan: Has diverse di- or oligomeric structures. + Is metabolized by intestinal bacteria
Lignin: Part of plant cell walls, difficult to degrade
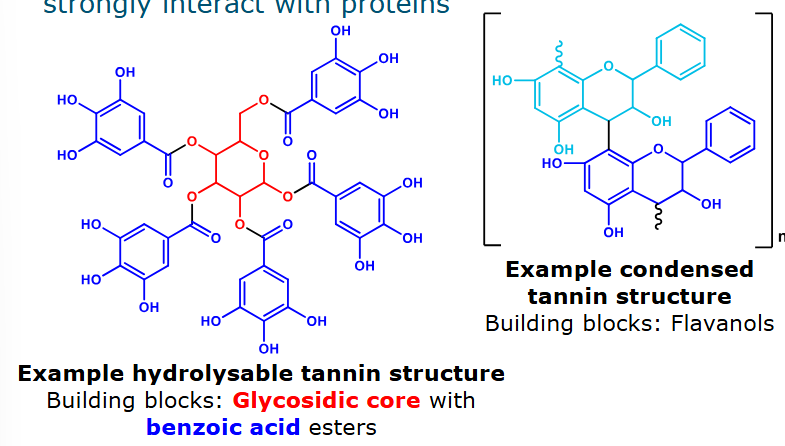
What are the two types of tannins?
Oligo- and polymeric phenolic compounds that strongly interact with proteins
Hydrolysable: hydrolysis releases gallic acid
Condensed: can be formed by oxidative coupling
flavonoids
Isoflavonoids
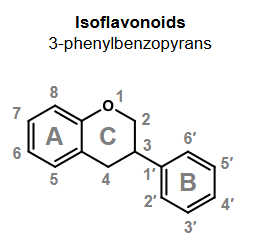
What reaction is desirable for many classes of phenolics?
Glycosylation
Makes phenolics more water-soluble and more stable
Glycoslyated phenolics are known as glycosides
The corresponding structure without glycosylation is known as the aglycon
Cis/Trans in hydroxycinnamic acids
In nature, the most abundant configuration is trans
the configuration can change during storage and processing of food or raw material
The trans and the cis hydroxy cinnamic acid have different properties
Phenolics as antioxidants
Radicals and metals can initiate undesirable reactions
Phenolic antioxidants can protect against oxidation
Flavonoids are often reported to be good antioxidants
Two ways of antioxidant activity in phenolics
Reducing oxidized compounds → e.g. by radical scavenging
Chelating metals = binding metal ions
Radical scavengers
Radical scavengers react with “free” radicals to form more stable, less reactive radicals
More resonance = better stabilized
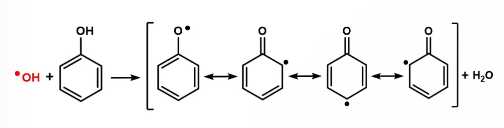
Desirable flavonoid structural features for radical scavenging
In general, additional OH groups
o-diphenol moiety on B-ring
C3 OH group on C ring
Antioxidant activity by metal chelation
O-diphenol moieties of phenolics bind metals → bound metals are less reactive
Certain other moieties can also bind metals
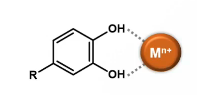
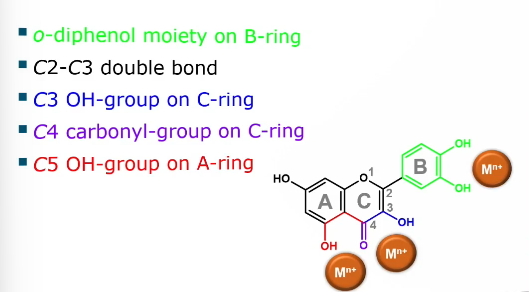
Desirable flavonoid structural features for metal chelation
o-diphenol moiety on B ring
C2-C3 double bond
C3 OH group on C ring
C4 carbonyl group on C ring
C5 OH group on A ring
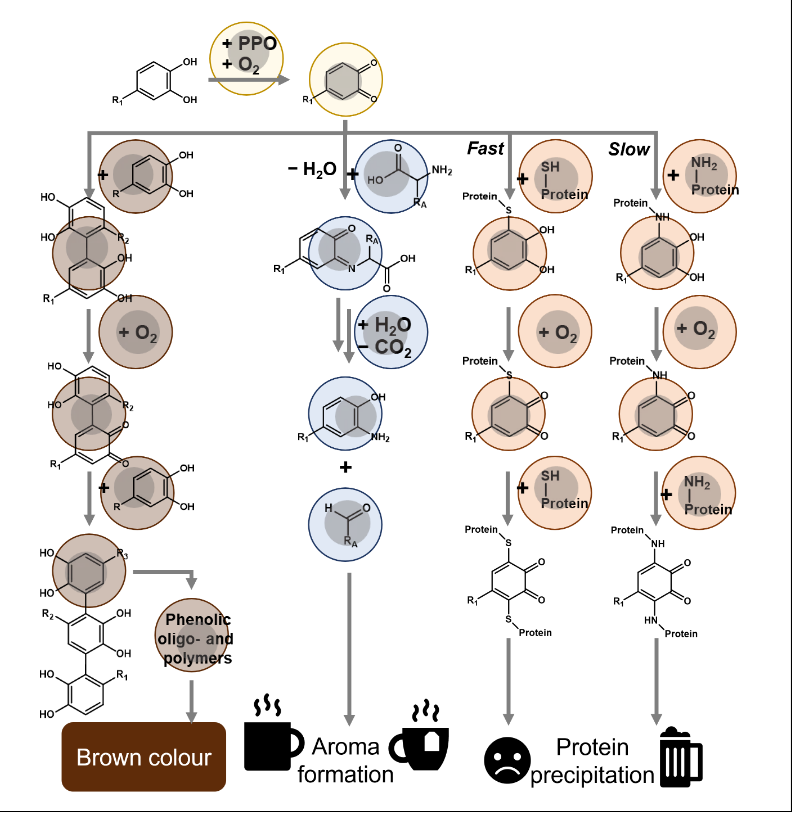
Enzymatic oxidation by polyphenoloxidase
Polyphenoloxidase (PPO) is an oxidative enzyme:
Phenolics as substrates
Oxygen as electron acceptor
Two copper ions in active site
Why does oxidation by PPO not happen when the plant is not damaged?
Because oxygen is needed for the oxidation reaction, which is not present in the plant cell
Therefore, when the plant becomes damaged oxidation by PPO can happen as there is access to oxygen.
PPO activity occurs upon damaging plant tissue during harvesting or processing
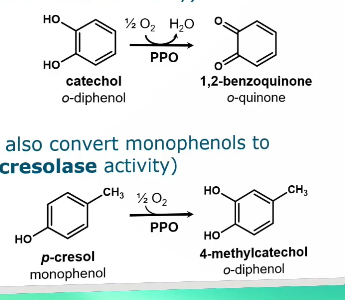
PPO converts phenolics to reactive electron deficient o-quinones (electron deficient structures)
Two ways that quinones are formed by use of PPO
PPO preferentially converts o-diphenols to o-quinones (= catecholase activity)
Some PPOs can also convert monophenols to o-diphenols (=cresolase activity)
The second reaction can lead back to the first reaction.
Monophenol → O-diphenol → o-quinone
Factors influencing formation of o-quinones
Overall PPO activity varies between sources
Characteristics and properties of the specific PPO
Structure of the phenolic compound
Conditions in the food process, ingredient, or product
Effect of o-quinone reactions on food properties
O-quinones are very reactive because they are electron deficient
O-quinones can therefore react with:
Other food molecules → leads to changes in flavor, color and appearance
Phenolic dimers → phenolic oligomers → phenolic polymers → insoluble brown pigments
The phenolic dimers and oligomers are soluble orange brown pigments and have interactions with proteins
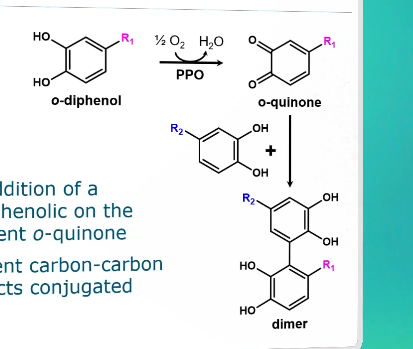
Initiation step of browning from o-diphenol
Nucleophilic addition of a non-oxidized phenolic to the electron-deficient o-quinone
Forms a covalent carbon-carbon bond → connects conjugated systems
This forms a dimer
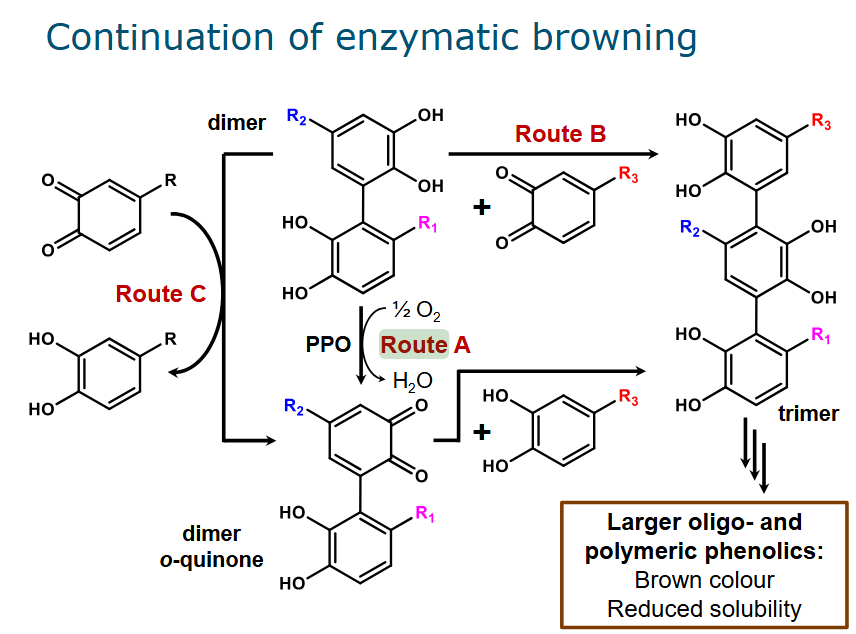
Three reactions to form brown pigment from dimer which was made from o-quinone
Route A: PPO acts on the dimer to form a dimer ortho-quinone
Route C: Coupled oxidation, meaning that an oxidized compound is reduced at cost of oxidizing another compound. This too forms dimer ortho-quinone
This can be coupled to route A, where a dimer is oxidized to a dimer ortho-quinone
The dimer o-quinone (no matter how it is formed) is turned into a trimer
Route B: o-quinone reacts directly with a dimer to form a trimer
The larger = more color and reduced solubility
How to control enzymatic browning
Eliminate oxygen
Lower pH (away from optimum for PPO)
Cool (lowers PPO activity)
Add chelating agents (bind to copper ions that PPO needs)
The above methods are not permanent (if temp increases, PPO will become active again). To completely inactivate PPO:
Heat-induced denaturation of PPO
Add ascrobic acid or other antioxidants
Add sulphite
Remove phenolics (using PVPPP followed by precipitation & filtration)
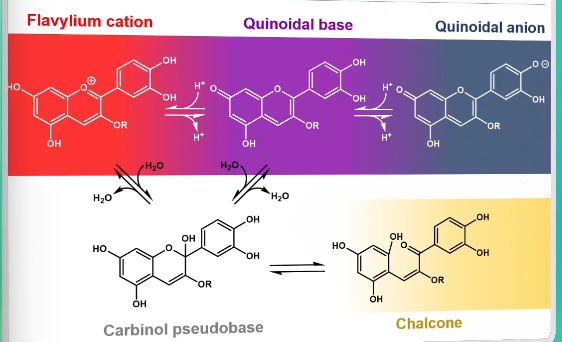
Anthocyanins
Flavonoids that have an extended conjugaed system → A and B ring connected via the C ring
pH dependent red-purple-blue color
pH dependent color of anthocyanins
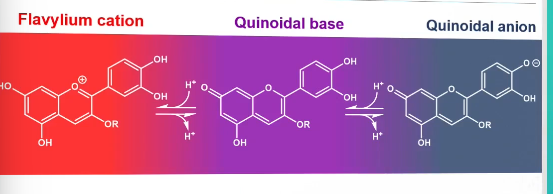
Addition of water to anthocyanins
The two types of protein-phenolic interactions
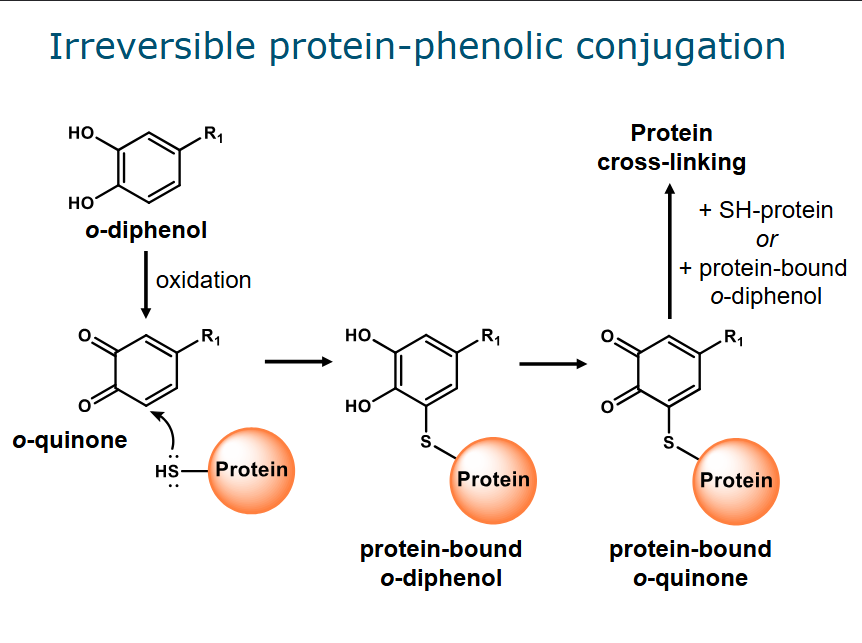
Protein-phenolic conjugates
Irreversible & covalent
Protein-phenolic complexes
Reversible & non-covalent
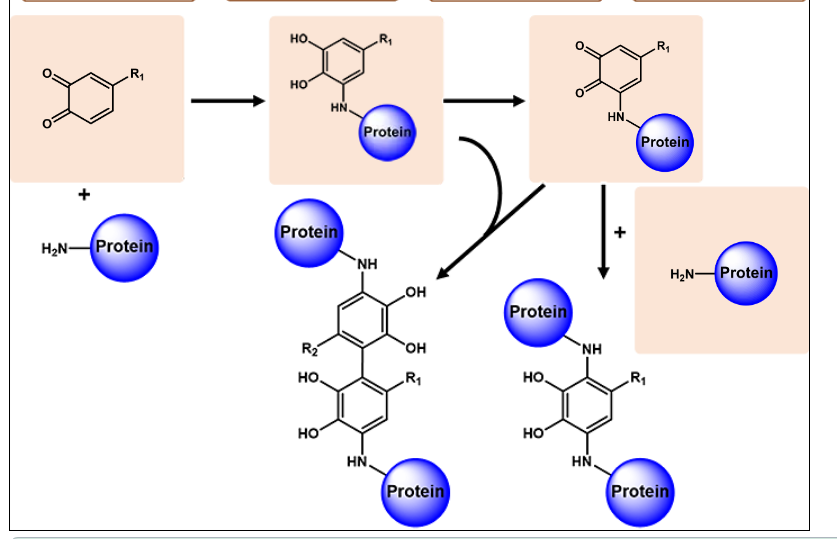
Protein-phenolic conjugation
Formation of o-quinones from o-diphenols by oxidation
Nucleophilic side chains in proteins attack the electron deficient o-quinones → protein bound o-diphenol
If this structure becomes oxidized again (→ protein bound o-quinone) it can be attacked by another protein or a protein bound o-diphenol creating a cross link (irreversible)
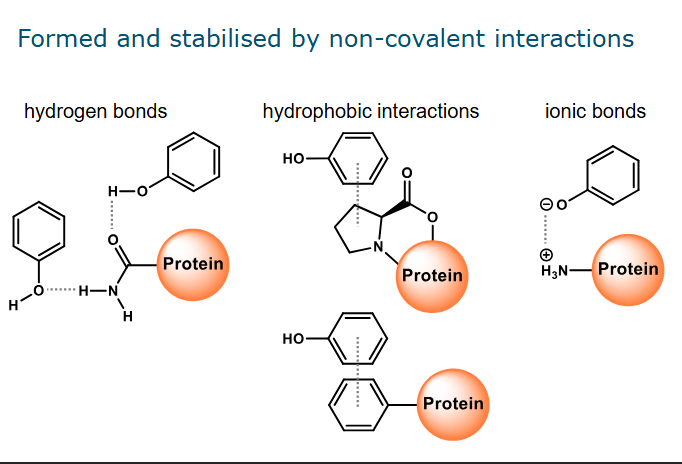
Protein-phenolic complexation
Hydrogen bonds - can be formed between OH group and a group in the amino acid side chain/the backbone
Hydrophobic interactions - take place between aromatic ring and hydrophobic groups/amino acid side chains
The main groups that play a role in these interactions are the ring structures of proline residues. And the aromatic side chains of tyrosine and phenylalanine residues.
Ionic bonds - a type of electrostatic interaction. Occurs mainly between deprotonated OH group and positively charged group in amino acid side chain.
Effect of phenolic/protein ratio
Low molar ratio: cross linking will likely happen (covalent or non-covalent or both). Causes aggregation and precipitation.
High molar ratio: proteins coated with hydrohpobic layer of covalently or non-covalently attached phenolics. Will also cause aggregation → precipitation
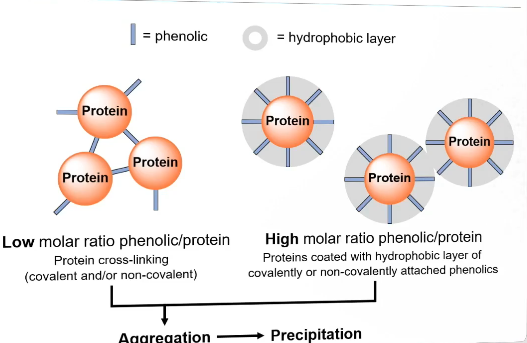
Preventing protein phenolic interactions
Removal of phenolic compounds
This can be done by reacting the phenolic to a proline rich protein or a protein analog (e.g. PVPP, strongly interacts with phenolics).
This will cause precipitation and makes the phenolic easy to remove.
Hydrolysis of (proline-rich) proteins → enzymatic hydrolysis with proteases. (prevents interactions with phenolics)
Controlling oxidation of phenolics → prevent formation of reactive o-quinones
Example phenolic protein interaction
Haze formation in beverages
Often considered to be undesirable
Caused by protein-phenolic complexes and conjugates with reduced protein solubility
Effect of addition of EDTA
Reversibly inactivates PPO by binding Cu2+
Effect of addition of ascorbic acid
Dual action: reduces o-quinone back to o-diphenol
Effect of addition of sulphite
Dual action: can bind to o-quinones and to the active site of PPO
Effect of blanching
Irreversibly inactivates PPO by denaturation
Effect of replacing air with nitrogen
Lack of oxygen prevents oxidation
Effect of addition of acetic acid
Reduces activity of PPO by moving away from optimum pH
What affects the color of anthocyanins
A reaction with water
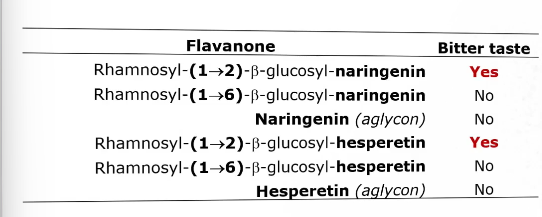
When do phenolics affect the taste of citrus fruit?
If they are present as glycosides
More specifically the taste-active forms are the (1→2) rhamnosyl-glucosides
When are condensed tannins most astringent?
At a DP of 5 to 7
Different flavanones and their bitterness
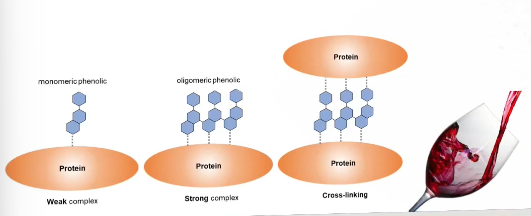
What makes phenolics astrigent?
Oligomeric phenolic compounds can strongly interact with proteins because they have multiple binding sites
They can even undergo cross linking
This will lead to aggregation and precipitation leading to an astrigent taste
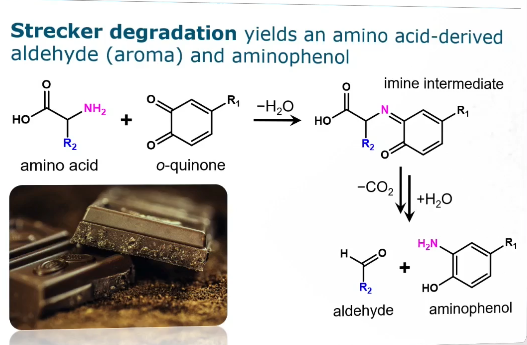
Strecker + what is its function
Strecker degradation yields an amino acid derived aldehyde (aroma) and aminophenol
Amino acid + o-quinone → imine intermediate → aldehyde + aminophenol
Desirable: The reaction produces highly odorous Strecker aldehydes (e.g., phenylacetaldehyde, methional) which contribute significantly to the desirable sensory properties and flavor profiles of many foods and beverages, such as chocolate and wine.
Undesirable: The same reaction can lead to the formation of off-flavors, contribute to the loss of beneficial antioxidants, and form potentially unstable intermediate compounds in certain contexts (like wine oxidation)
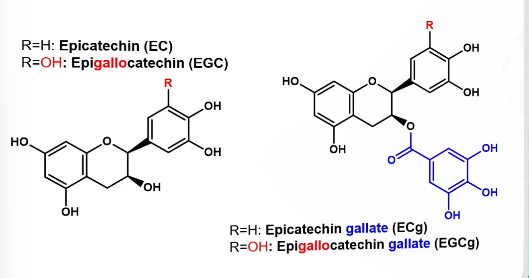
Phenolics in tea
Tea leaves are rich in phenolics (20-30% DM) → mainly catechins
Most importantly epicatechin gallate and epigallocatechin gallate
A type of flavanol
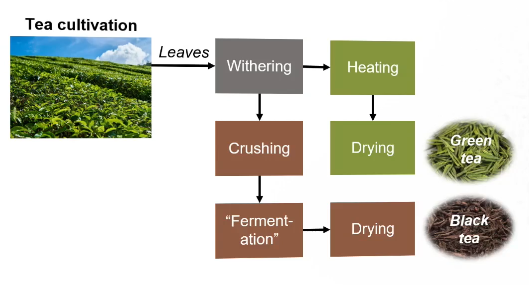
The tea production process (green & black tea)
Black tea is crushed because it exposes the leaves to air → causing oxidation and allowing PPO to work
This oxidation step is written in the slide as “fermentation”
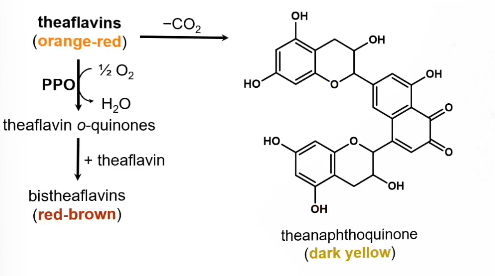
Follow up reaction of theaflavins
Process of theaflavin & bisthea flaving production
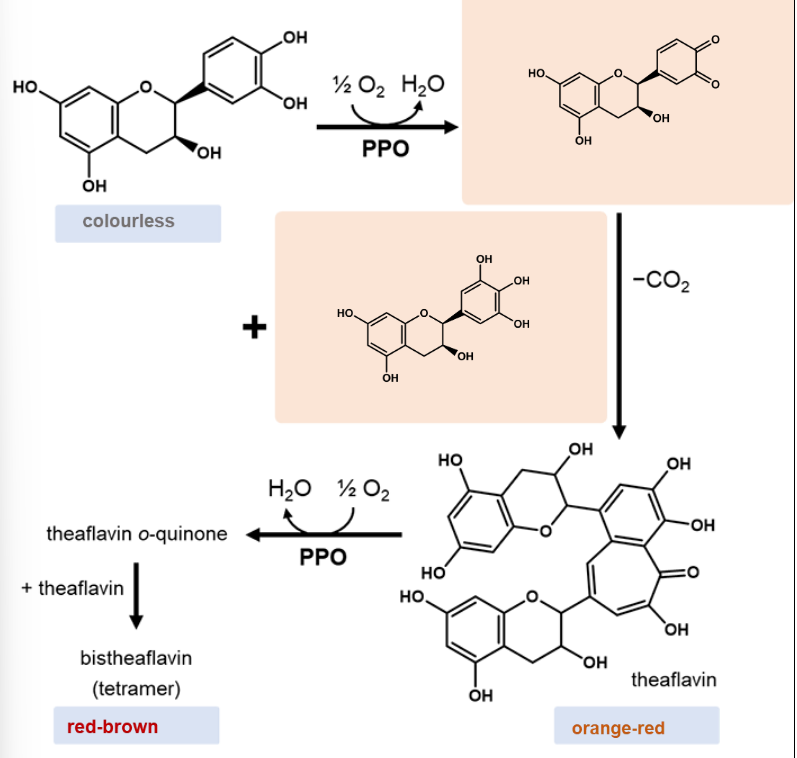
All reactions
Why should you not use “polyphenols”?
The prefix “poly” in the name polyphenol refers to the multiple hydroxyl groups that often occur in the structures of phenolic compounds. However, many common phenolics, such as p-coumaric acid and p-hydroxybenzoic acid, only possess one hydroxyl group on an aromatic ring.
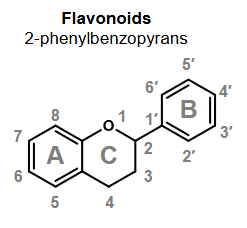
What do flavonoids collectively refer to?
2-phenylbenzopyrans
3-phenylbenzopyrans
Detection of phenolic compounds by visual observation
By increasing the pH of e.g. a juice, you can tell if there are anthocyanins.
If the juice turns from red to purple to blue and eventually yellow it is highly likely that anthocyanins are present.
Detection of phenolics by UV-Vis spectrophotometry
The absorbance of UV and visible light by compounds in solution can be measured.
This works very well for phenolics because they always have at least one aromatic ring, which absorbs light in the UV range.
Larger phenolics = absorb light in the visible light range
Additionally, there are many colorimetric assays that rely on a reaction that results in a change in the absorbance of light of a specific wavelength.
How to quantify total amount of phenolics
Folin-Ciocalteu assay
Which is a colorless mixture of two metals that can oxidize phenolic compounds and in the process the metals become reduced.
Their reduced form has a bright blue color, that can be visually observed an dmeasured by UV-Vis spectrophotometry.
Total Phenolic content (TPC), is often expressed as gallic acid equivalents.
How to measure antioxidant activity?
There are different ways
Most of which are colorimetric assays that measure radial scavenging activity
Not very reliable and may be interfered by other compounds present in the sample.
Advanced methods for analysis of phenolics
Most commonly used: combination of liquid chromatography with detection by UV-Vis spectrophotometry and/or mass spectrometry.
More accurate and less interfered by other compounds + gives info on structure of phenolic.
Downside: expensive, takes time, more complicated.
Why are hydroxycinnamic acids and hydroxycinnamyl alcohols nature’s building blocks?
Hydroxycinnamic acids in particular are abundant in nature in their free form. Moreover, their carboxylic acidgroup allows them to form esters or amides with molecules that possess alcohol (–OH) or amine (–NH2) groups.
Hydroxycinnamic acids as well as hydroxycinnamyl alcohols serve as building blocks for many other types ofphenolic compound
Which phenolics are responsible for astringency?
Condensed and hydrolysable tannins