VSEPR Theory and Molecular Geometry
1/22
Earn XP
Description and Tags
Put the Name and bond angle for example "Tetrahedral, 109.5" also 1 unshared is the same as 1 lone pair
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
4 σ Bonds
Tetrahedral, 109.5
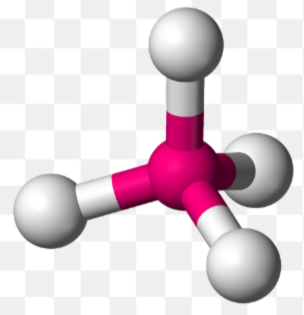
Tetrahedral, 109.5
3 σ Bonds & 1 lone pair
Trigonal Pyramidal, 107
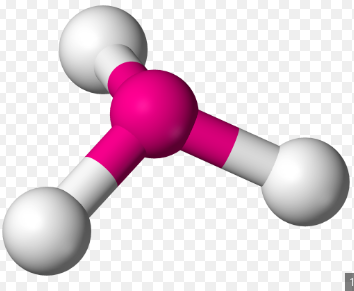
Trigonal Pyramidal, 107
2 σ Bonds & 2 lone pairs
Bent, 104.5
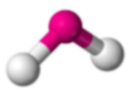
*2 Lone Pairs*
Bent, 104.5
3 σ Bonds
Trigonal Planar, 120
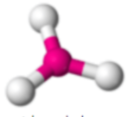
Trigonal Planar, 120
2 σ Bonds & 1 Lone Pair
Angular, 117
2 σ Bonds
Linear, 180
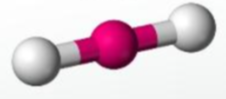
Linear, 180
5 σ Bonds
Trigonal bipyramidal, 120 and 90
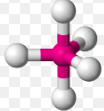
Trigonal bipyramidal, 120 and 90
6 σ Bonds
Octahedral, 90
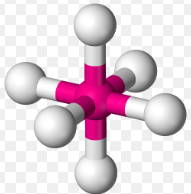
Octahedral, 90
5 σ Bonds & 1 Lone Pair
Square pyramidal, 90
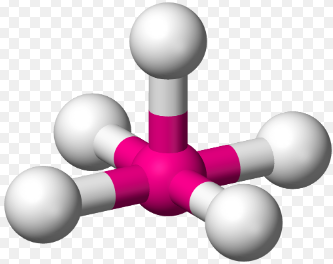
Square Pyramidal
4 σ Bonds & 2 Lone Pairs
Square planar, 90
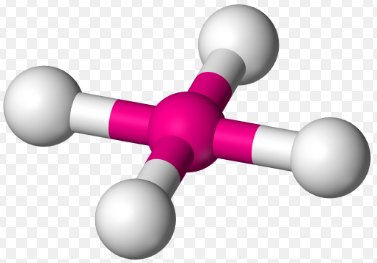
Square Planar, 90
4 σ Bonds & 1 Lone Pair
See-saw, 87 and 172
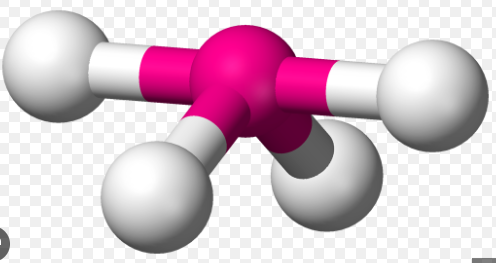
See-saw, 87 and 172
3 σ Bonds & 2 Lone Pairs
T-shaped, 87 and 172
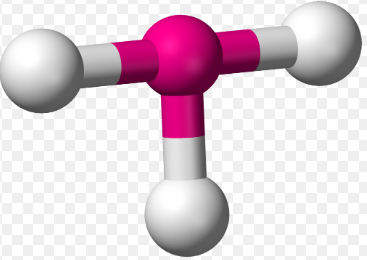
T-shaped, 87 and 172