C1 - Atomic Theory
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Structure of an atom
Consist of three subatomic particles: protons, neutrons and electrons
Nucleus of an atom
Contains protons and neutrons
Has a positive charge and is very small and dense
Contains most of mass
Electrons in an atom
Negatively charged
Orbit the nucleus in shells/orbitals
Most of atom is made of empty space
Properties of subatomic particles
Protons
RM: 1
RC: +1
Neutrons:
RM: 1
RC: 0
Electrons
RM: 1/1840
RC: -1
Mass number
Total number of protons and neutrons in the nucleus
Bigger number
Atomic number
Total number of protons in the nucleus, and defines the element
Isotope
Different atoms of the same element which have same number of protons and electrons but a different number of neutrons
Means they have a slightly different physical property (mass and density)
Same chemical properties (reactivity)
Relative isotopic mass
Mass of an atom of an isotope compared to 1/12 of the mass of carbon-12
Rounded to one dp
Relative atomic mass
Mean mass of an atom of an element compared to the mass of 1/12 of carbon-12
Calculated by (sum of (ab x mass)) / sum of ab
Relative molecular mass (Mr)
Used for simple covalent molecules
Calculated by adding the Ar values of all atoms in one molecule
Relative Formula Mass
Used for ionic compounds
Used for giant structures
Calculated by adding all the Ar values of all the ions in one formula unit
Emission spectra
Electrons absorb and release energy in discrete amounts
Electrons occupy fixed energy levels called quantum shells
They can be excited to higher energy levels by absorbing energy or vice versa
Lines in emission spectra
Display the frequencies of light emitted as electrons transition from higher/lower energy levels
Quantum shell model (and how this provides evidence for quantum shells)
Electrons are confined to fixed shells
Each shell has a defined energy and electrons cannot exist between shells
Electrons must absorb/emit electromagnetic radiation to move
Emitted radiation has fixed frequencies
How does emission spectra support the Quantum Shell Model
Electrons cannot smoothly transition
Presence of distinct lines rather an a spectrum indicated quantized energy levels
Each line represents a specific electronic transition
Mass spectroscopy
Plots the relative abundances of ions against their mass to charge ratio (m/z)
X axis:
m/z values equals relative mass of ion
Y - axis:
Relative abundance
Mass spectroscopy: molecular samples
Peak at highest m/z value is the molecular ion (M+)
This is the molecular ion peak
Matches the molecule’s Mr
Lower peaks come from fragments of molecular ions
Ionisation energy
Refers to the process of removing one or more electrons
Endothermic reaction
First Ionisation energy
Energy needed to remove 1 electron from each atom in one mole of gaseous atoms to form 1 mole of gaseous 1+ ions
Factors that affect ionisation energy
NASA
Nuclear Charge
Atomic Radius
Shielding effect
How this impacts Nuclear Attraction
Nuclear Attraction
More protons = stronger positive charge = stronger electrostatic force of attraction
Atomic Radius
Electrostatic attraction drops off steeply with increasing distance
Electrons in smaller atoms are held closer so attraction is greater
Shielding effect
Inner electrons create shielding effect for outer electrons
More electrons means more shielding
Trends in ionisation energy: Group
Decreases down a group
Nuclear charge increases down as more protons are added
Increases attraction for electrons
Atomic radius increases as more electron shells are added
Electron shielding increases down a group as more inner shells reduce nuclear attraction
Trends in ionisation energy: Period
Increase across a period
Nuclear charge increases as more protons are added across a period
Atomic radius decreases across a period as more electrons are added
Electron shielding stays similar across a period
Successive ionisation energies
(Energy needed to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous ions) needed to remove each successive electron
Large jumps
When entering a new shell, there are often large jumps because nuclear attraction increases greatly
General pattern before is that energy increased
FIE of Na
Na(g) —> Na+(g) + e-
5th Ionisation Energy of Na
Na4+(g) —> Na5+(g) + e-
Shells
Hold electrons
Each one is defined by a principal quantum number, indicating the relative distance
Is divided into sub-shells
Sub-shells
Have distinct energy levels labelled s, p, d
Contain orbitals
Electrons in subshells
s: can have 1 orbital and therefore 2 electrons
p: can have 3 orbitals and therefore 6 electrons
d: can have 5 orbitals and therefore 10 electrons
Orbitals
Are regions with a high probability of finding an electron found around the nucleus
Can accommodate up to 2 electrons with opposite spins
S orbital
O shape
P orbital
Dumbbell shape
Electrons in the first four shells
Electrons in the first four shells
Shell 1: 1s, 2 electrons
Shell 2: 2s and 2p, 8 electrons
Shell 3: 3s and 3p and 3d, 18 electrons
Shell 4: 4s, 4p, 4d, 4f and 32 electrons
Subshell notation
Sub-shell notation
Indicated the number of electrons within each subshell
1s2 2s2 2p6
Working out electron configuration
Fill out the lowest energy orbitals first
Fill subshells singly before pairing up
Must have opposite spins
For ions in s/p blocks, electrons are added to/removed from highest occupied subshell
Order of subshells
1s
2s
2p
3s
3p
4s
3d
4p
Noble gas notation
Use square brackets of the closest preceding noble gas
e.g [Ar] 4s2
Chromium notation
1s2 2s2 2p6 3s2 3p6 3d5 4s1
Copper notation
1s2 2s2 2p6 3s2 3p6 3d10 4s1
Why are copper and chromium irregular
These are irregular because configurations with d subshells are energetically more favourable
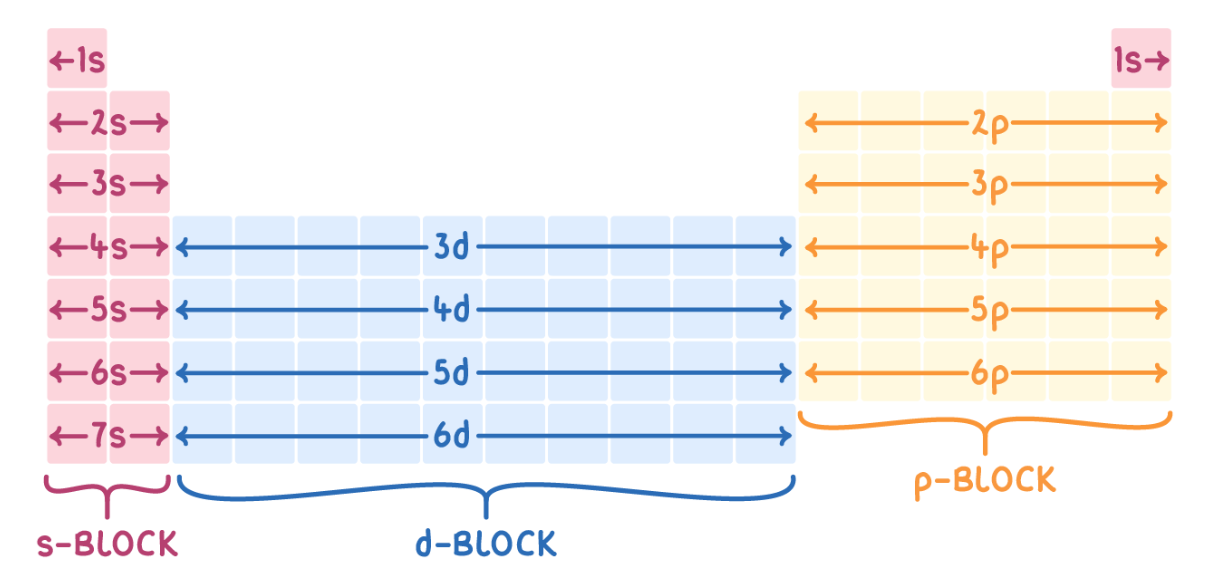
Arrangement of periodic table
In order of increasing atomic number
Can predict their behaviour
Divided into groups and periods
s/p/d blocks
Have their valence electron in that orbital
Periodic properties
Electronic configuration determines the chemical properties
Type of bonds and their strength will affect the melting/boiling points of elements in period 2 and 3
Metals properties due to e config
Strength of metallic bonding increases across the period
Ions have larger positive charge and smaller ionic radius
More delocalised electrons
Means greater electrostatic force of attraction
Giant covalent lattices properties due to e config
Carbon and silicon
A huge amount of energy is required to break the covalent bonds so very high bp/mp
Simple covalent properties due to e config
Only have weak induced dipole forces existing between molecules which are very easy to overcome
Noble gases properties due to e config
Very low bp/mp as no bonds formed and very weak induced dipole-dipole forces attract
First Ionisation Energy periodicity in the periodic table
Rules as before apply BUT there is a variation in drops between group 2-3 and 5-6
Why are the rules different for groups 2 - 3
In group 3 the electron in removed from p orbital rather than s like in group 2
P orbitals have slightly higher energy than s orbitals so valence electron is an average further
P orbital also experiences additional shielding from nucleus by s electrons
Means less energy is required