Lesson 3 Protein Synthesis and Trafficking in Neurons
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
Why is the movement of proteins within a neuron important?
Because it is essential to neuronal function and signaling.
What structural characteristic makes neurons unique?
Neurons are highly polar, have highly organized cellular structures and compartments that serve different functions.
What role do proteins play in forming neuronal structures?
They form many cellular constituents (molecules) of the cell itself, such as microtubules.
What role do proteins that catalyze reactions play in neurons?
They act as enzymes that catalyze various reactions, including the synthesis of neurotransmitters in different organelles.
How do proteins contribute to signaling in neurons?
They act as membrane cell-surface markers and receptors for signaling, such as G-protein–coupled receptors.
How do proteins help move substances across membranes?
They serve as integral membrane channels and pumps, such as the sodium-potassium pump
Proteins are composed of
amino acids
Besides building proteins, what other role can amino acids serve?
energy metabolites, acting as precursors of glucose, fatty acids, and ketone bodies.
nonessential amino acids can be synthesized from
metabolic precursors
Essential amino acids must be obtained from
the diet
What happens to excess dietary amino acids?
They are not stored or excreted; they are converted to metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-ketoglutarate.
Why are most amino acids called α-amino acids?
Because they have a central α-carbon.
What groups are attached to the α-carbon in most amino acids?
A primary amino group and a carboxylic acid group, except in proline.
What makes one amino acid different from another?
The “R” group, which varies among amino acids.
What happens to the amino and carboxyl groups of amino acids at physiological pH?
Both groups are completely ionized.
Why are amino acids considered amphoteric?
Because they can act as either an acid or a base.
How are the various proteins in neurons formed?
Different amino acids combine to form the various proteins found in neurons.
How are amino acids joined together to form proteins?
through the elimination of a water molecule, creating a CO-NH linkage to form a peptide bond.
What results from joining many amino acids by peptide bonds?
polypeptides.
What are proteins composed of?
one or more polypeptide chains, which can range from about 40 to 34,000 amino acid residues in length.
How many levels of protein structure exist, and what do they represent?
There are four levels of protein structure, ranging from the primary amino acid sequence to complex 3-D shapes found in cell membranes.
What is the primary structure of a protein?
the amino acid sequence.
What is the secondary structure of a protein?
alpha helices and beta-pleated sheets.
What is the tertiary structure of a protein?
occurs when α-helical or β-pleated regions fold upon one another to form a 3-D shape.
What is the quaternary structure of a protein?
formed when two or more polypeptide chains combine.
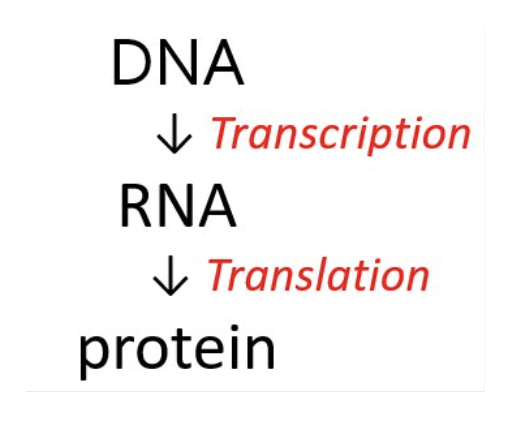
What is transcription?
the synthesis of RNA (all types of RNA) from the genetic code in DNA.
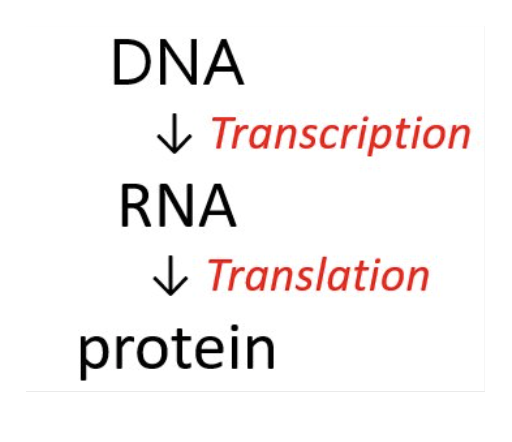
What is translation?
the synthesis of proteins from amino acids.
What determines which amino acid is added to a growing peptide?
Codons in RNA
How are bases in mRNA read during translation?
in triplets called codons, which determine which amino acid is added to the polypeptide chain.
How many possible codon combinations exist?
There are 64 possible codon combinations (4³ = 64).
What is the function of the codon AUG?
AUG codes for methionine (Met) and can also function as the “start” signal.
Which codons function as stop codons?
UAA, UAG, and UGA
Where does protein synthesis occur, and how are codons read?
on ribosomes, where tRNAs read each codon.
What does each codon in mRNA code for, and how is it carried?
a specific amino acid, which is carried by a tRNA molecule.
How does the ribosome build a polypeptide chain?
As the ribosome reads the mRNA strand, each incoming tRNA will add a particular amino acid to the growing polypeptide chain.
What determines the sequence of amino acids in a protein?
the sequence of bases in the triplet (the codon) (i.e., the anticodon sequence in the tRNA is complementary to the sequence in the mRNA strand- the codon)
When does translation stop?
Translation continues until a stop codon is encountered.
How is the nuclear membrane related to the endoplasmic reticulum (ER) membrane?
The nuclear membrane is continuous with the endoplasmic reticulum (ER) membrane.
How is the nuclear membrane connected to the endoplasmic reticulum (ER)?
The double membrane of the nucleus is continuous with the membrane of the endoplasmic reticulum (ER).
What feature does the outer nuclear membrane share with the rough ER?
Like the rough ER, the outer nuclear membrane can be covered with ribosomes.
Where are proteins synthesized on the outer nuclear membrane transported?
Proteins synthesized here are transported into the perinuclear space, the space between the inner and outer nuclear membranes which is continuous with the lumen of the ER.
What happens to tRNAs and mRNAs synthesized in the nucleus?
They can be exported to the cytosol.
Where are proteins made in the cell?
They are made in the cytosol and many remain in the cytosol after synthesis
How do proteins made in the cytosol reach the nucleus, and which proteins are transported this way?
Bidirectional traffic between the nucleus and cytosol allows proteins made in the cytosol—such as histones, DNA and RNA polymerases, gene regulatory proteins, and RNA-processing proteins—to be imported into the nucleus.
Which proteins made in the cytosol are imported into the nucleus?
Histones, DNA and RNA polymerases, gene regulatory proteins, and RNA-processing proteins.
Where are most of a cell’s proteins made, and are there exceptions?
Most proteins are made by ribosomes on the ER, but some proteins are synthesized on free-floating ribosomes not associated with the ER
Can proteins also be synthesized on ribosomes not associated with the ER?
Yes, some proteins are made on free-floating ribosomes in the cytosol.
Where are many proteins further processed, and what type of proteins undergo this processing?
Many proteins, including secretory and membrane proteins, are further processed in the lumen of the ER as part of posttranslational modifications.
What primarily determines the fate of a protein?
Its amino acid sequence (the primary structure of a protein).
Where do most proteins remain after synthesis?
In the cytosol, where they perform various cellular functions.
When can proteins be modified?
During and/or after synthesis.
why are proteins modified?
for a variety of reasons, such as proper folding, stability, localization, and function.
How can proteins be modified once they are made?
By various enzymes either during synthesis (cotranslational modification) or after synthesis (posttranslational modification).
What are modifications that occur during synthesis called?
Cotranslational modifications
What are modifications that occur after synthesis called?
Posttranslational modifications.
What is an example of a posttranslational modification?
disulfide linkages (Cys-S-S-Cys).
How are disulfide linkages formed?
Through oxidation (loss of electrons) of free sulfhydryl side chains on cysteine residues.
Why are disulfide linkages important?
They are critical to the tertiary structure of proteins.
Why can’t disulfide linkages form in the cytosol?
Because the cytosol has a reducing environment (it provides electrons), which prevents the oxidation needed for disulfide bond formation.
How are proteins moved efficiently over short distances in neurons?
By diffusion, which works well for moving macromolecules throughout the cell body and to the proximal ends of dendrites and axons.
Why is protein movement to distal dendrites and axons more challenging?
Because they are far from the soma, requiring active transport and energy expenditure.
What obstacle must proteins face when being inserted into or exported across the cell membrane?
The cell membrane’s structure—its hydrophobic interior and hydrophilic surfaces—makes protein insertion difficult. On both the intracellular and extracellular surfaces
How do transmembrane proteins overcome this obstacle?
They contain hydrophobic domains that embed within the phospholipid bilayer and hydrophilic domains that remain exposed on intracellular and extracellular surfaces.
How far can translational diffusion effectively move proteins in cells?
only for short distances at best
What is diffusion?
the random movement of particles or molecules, partly due to random collisions.
How does diffusion in cells differ from diffusion in a dilute environment?
In cells, diffusion occurs in a complex environment with macromolecular crowding, viscosity, physical barriers, and specific or nonspecific binding, all of which affect distance and rate of travel.
How does protein size affect diffusion?
Diffusion rate is inversely proportional to protein size—larger proteins diffuse more slowly.
How does diffusion of proteins in membranes compare to the cytoplasm?
It is much slower
Does the charge of proteins affect diffusion?
Yes, proteins vary in charge as well as size, and this can influence their diffusion rates.
How are proteins directed to their correct cellular location or compartment?
Signal peptides and signal patches direct proteins to the correct location and/or compartment.
How many types of sorting signals exist on proteins?
There are at least two types of sorting signals on proteins.
What is a signal peptide?
it is a continuous stretch of amino acids, typically 15–60 residues long, that directs proteins to specific locations. It is often (but not always) removed from the finished protein after sorting.
What is a signal patch?
is a specific three-dimensional arrangement of atoms on the protein’s surface formed when the protein folds. Amino acids in a signal patch can be distant from one another in the linear amino acid sequence, and they generally remain in the finished protein.
What are signal peptides used for?
They direct proteins from the cytosol into organelles such as the ER, mitochondria, chloroplasts, peroxisomes, and nucleus, and help retain soluble proteins in the ER.
What are the three main mechanisms by which proteins move between compartments?
(1) Gated transport, (2) Transmembrane transport, and (3) Vesicular transport.
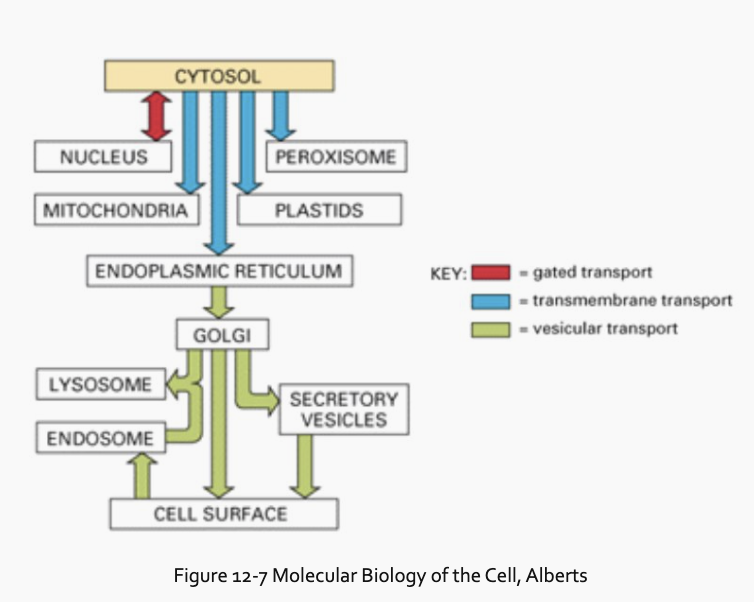
What is gated transport and where does it occur?
between compartments with continuity of their spaces, such as between the nucleus and the cytosol. Nuclear pores act as gates for larger molecules, while smaller molecules can pass by diffusion.
What is transmembrane transport and how does it work?
between two distinct compartments, such as from the cytosol into the ER or mitochondria. Proteins are directed by a membrane-bound translocator and usually must be unfolded to pass through the membrane.
What is vesicular transport and how does it work?
it uses transport vesicles to ferry proteins from one compartment to another, such as the transfer of soluble proteins from the ER to the Golgi. The vesicle membrane fuses with the target membrane to deliver the protein.
How are signal peptides and signal patches recognized?
They are recognized by complementary receptor proteins in the target organelle.
How does a newly synthesized organelle protein find its way from the ribosome where it is made to the organelle where it functions?
It follows a specific pathway guided by signals in its amino acid sequence (signal peptides or signal patches).
Do proteins that function in the cytosol contain signal peptides or patches?
No, cytosolic proteins lack signal peptides or patches and remain in the cytosol after synthesis.
Does vesicular transport of proteins from the ER through the Golgi to the cell surface require specific sorting signals?
No
Why are specific sorting signals needed for the ER and Golgi?
They are needed to retain specialized resident proteins
What is required for the transport of large protein molecules into the nucleus?
ATP
How are protein particles and organelles moved?
They are actively transported along axons and dendrites.
What types of movement occur along axons?
Both fast and slow axonal transport occur.
What is transported by fast axonal transport?
Organelles and vesicles are transported by fast axonal transport, in both anterograde (toward the axon terminal) and retrograde (toward the soma) directions.
What is transported by slow axonal transport?
Cytoskeletal elements (microtubule subunits, neurofilaments, actin) and various soluble proteins are transported by slow axonal transport, typically in the anterograde direction.
Which molecular motors are critical for protein transport in neurons?
Kinesin and dynein
What motor protein aids anterograde fast transport (about 400 mm/day) along microtubules?
Kinesin, an ATPase, aids anterograde fast transport, moving toward the plus end of microtubules, away from the cell body.
What happens to kinesin-mediated transport if microtubules are disrupted?
Transport function is lost if the microtubules are disrupted.
Which motor protein aids fast retrograde transport?
Dynein, an ATPase, moves toward the minus end of microtubules, opposite to kinesin.
What is the effect of ATP loss on axonal transport?
Loss of ATP results in the loss of both anterograde and retrograde fast transport.
Exocytosis moves substances
out of the cell
Endocytosis moves substances
into the cell.