chemistry - measurements
1/27
Earn XP
Description and Tags
probably wont contain all the notes/lab questions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
motto to remember measurements
king
henry
died
unexpectedly
drinking
chocolate
milk
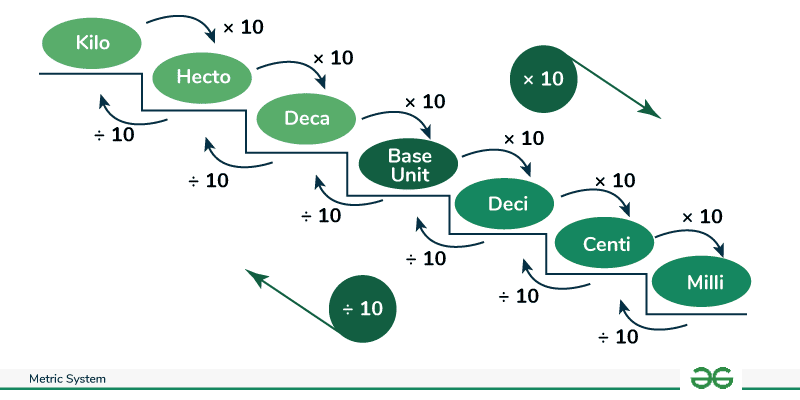
actual measurements for motto
KILOmeters
HECTOmeters
DECAmeter
unit (mL, L,…)
DECImeters
CENTImeters
MILImeters
accuracy
evaluation of how close a measurement is to the acceptable value of the measurement
precision
evaluates how exactly a measurement is made
SigFig Rule #1
SigFigs only apply to measured data
not counted #s, pure #s, or fractions like 60 secs/1 min
SigFig Rule #2
all NONZERO digits are significant
112.54 - 5 sigfigs
rule #3
all ZEROs between NONZEROS digits are significant (sandwiched)
10.6 - 3 sigfigs
rule #4
decimal points define Significant 0s
if a decimal point is present, all zeros to the ____ of the last nonzero digits are significant
right
when are zeros not significant
no decimal present
no zeros to the LEFT of the FIRST nonzero digit
.050 - 2 sigfigs
if a decimal is not present, the trailing zeros are _____
not significant
rule #5
a decimal must follow and estimated zero in ones place
if estimated value of measurement is 0 in ones place, it’s impossible tot tell precision
when adding/subtracting sigfigs, determine the least amount of ____
decimal places, give answer in least amount of given decimal places
12.3456 + 3.8 = 16.1456 = 16.1
when multiplying/dividing sig figs, determine SMALLEst # of ____
sigfigs, give answer in same lowest amount of sigfigs
34.6 × 2 = 69.2 = 70
46.9000 / 3.3 = 14.2121 = 14
quantitive data
numerical data
discrete (counting)
continuous (measurement)
qualitive data
descriptive data based on observations (words)
involves 5 senses
metric prefixes
what do sigfigs do
help scientist determine precision of instrument
Things we measure when we measure
amount of matter
mass
volume
density
weight is measured in ____
newtons
1 lb is = ___ N (newtons)
4.4482216 N
mass is the measure of the amount of ___ in a substance
matter (g/kg)
volume is the amount of ___ an object occupies
space (mL/L)
what is density measuring?
the amount of mass per volume (kg/m squared or g/cm squared)
density = mass/volume
water displacement (volume measuring method)
place object in graduated cylinder with marked volume
add object and read the change in volume with object and subtract to find the volume of the object