module 5 - physical chemistry & transition elements
1/129
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
130 Terms
What is lattice enthalpy?
The lattice enthalpy of formation is the enthalpy change when one mole of an ionic lattice/compound is formed from its gaseous ions under standard conditions
The lattice enthalpy change of dissociation is when gaseous ions are dissociated from one mole of an ionic compound/lattice under standard conditions.
What is lattice enthalpy a measure of?
Strength of ionic bonding in a giant ionic lattice
Is lattice enthalpy endo/exothermic and why?
Exothermic - ionic bonds form between separate gaseous ions, releasing energy
Why are solid ionic compounds stable?
The strength of ionic bonds creates a substantial energy barrier that must be overcome to break down the ionic lattice
How is lattice enthalpy determined and why?
By the use of a Born-Haber cycle as lattice enthalpy cannot be measured directly/ practically using calorimertry etc
What is enthalpy change of formation?
The enthalpy change when one mole of compound is formed from its constituent elements under standard conditions, all reactants and products being in their standard states
What is enthalpy of atomisation?
The enthalpy change when one mole of gaseous atoms is formed from the element in its standard state e.g. ½ Cl2(g) → Cl(g)
Is enthalpy of atomisation endo/exothermic and why?
Endothermic - bonds are broken between atoms, requiring energy
What is first ionisation energy?
The first ionisation energy is the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions
Is first ionisation energy endo/exothermic and why?
Endothermic - energy is required to remove an outer electron from the atom as the negative electron is attracted to the nucleus
Why is second ionisation energy more endothermic than first ionisation energy?
There are the same number of protons acting on less electrons so each electron experiences a greater attraction from the nucleus - more energy is needed to overcome the attraction.
What is first electron affinity?
The enthalpy change when one electron is added to each atom in one mole of gaseous atoms to form 1 mole of gaseous -1 ions
Is first electron affinity endo/exothermic and why?
Exothermic - an atom with an electron affinity will “want” to gain an electron to fill its valence shell so easily gains this electron
Is second electron affinity endo/exothermic and why?
Endothermic - the negative electron being added is repelled by the negative ion it is being added to/the added electron that is already there as electrons repel each other - so energy is required
Why is the first electron affinity for chlorine more exothermic than for bromine?
Cl has a smaller atomic radius and its outer shell experiences less shielding even though Br has a greater nuclear charge
So greater nuclear attraction in Cl than Br with outer electron being added, releasing more energy
What are the two routes involved when measuring lattice enthalpy?
Route 1: direct formation of the ionic compound from its elements -Enthalpy of formation
Route 2: elements form gaseous atoms - atomisation, electrons are added/removed - ionisation energies and electron affinities to form gaseous ions, then the ionic compound forms - lattice enthalpy.
How is Hess' Law used to measure lattice enthalpy?
Route 1 = Route 2
ie. ∆H.f = ∑∆H.atom + ∑∆H.ionisation + ∑∆H.electron affinity + ∆H.LE
What is the order of enthalpies when measuring lattice enthalpy?
Atomisation always precedes ionisation energy
Atomisation always precedes electron affinity
Ionisation energy comes before electron affinity
Lattice enthalpy comes last
What factors affect lattice enthalpy?
Ionic size/radius - the smaller the large LE
Ionic charge - the larger the larger LE
How does ionic radius affect lattice enthalpy?
Smaller ionic radius makes lattice enthalpy more exothermic
Ions can pack closer together, leading to greater electrostatic attraction
Electrostatic attraction between ions is stronger so stronger ionic bonds
Higher charge density
More energy is released when bonds form & more energy is required to overcome the bonds to break them
How does ionic charge affect lattice enthalpy?
Greater ionic charge makes lattice enthalpy more exothermic
Electrostatic attraction between ions is stronger so stronger ionic bonds
Higher charge density
More energy is released when bonds form & more energy is required to overcome the bonds to break them
What effect does lattice enthalpy have on melting point?
Typically, more exothermic lattice enthalpy results in higher melting point
How are exothermic and endothermic reactions shown on a Born-Haber cycle?
On a Born-Haber cycle, species of higher energy are closer to the top so endothermic reactions point upwards and exothermic reactions point downwards
What does the Born-Haber cycle for LiF look like (lattice enthalpy)?
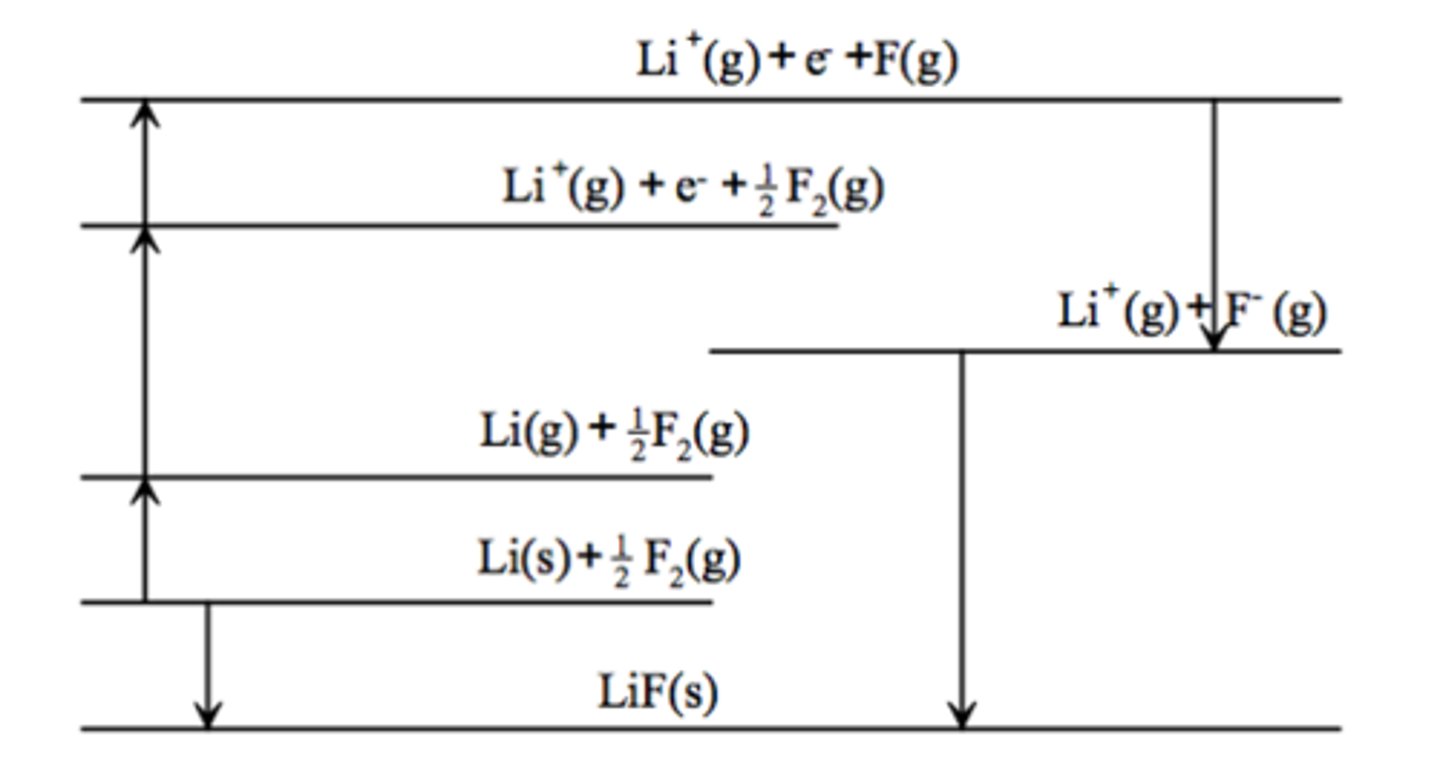
What is important to remember when drawing Born-Haber cycles?
Between each horizontal energy level, only one species changes and all the species are balanced
Highest energy is at the top
What is standard enthalpy change of solution?
Enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution
What is required for a substance to dissolve?
Bonds between the substance must be broken - endothermic
New bonds must be formed between the substance and solvent - exothermic
What must the bonds in solution be like for a substance to be soluble in a particular solvent?
The new bonds formed between the substance and solvent must be the same strength or greater than those broken in the substance.
If this doesn’t happen, the substance is very unlikely to dissolve. Soluble substances tend to have exothermic enthalpies of solution for this reason.
What is standard enthalpy change of hydration?
Enthalpy change that takes place when one mole of aqueous ions is made from one mole of gaseous ions under standard conditions.
What are the two routes involved when measuring enthalpy of solution?

Route 1: aqueous solution forms from gaseous ions dissolving (enthalpy of hydration)
Route 2: ionic compound forms (lattice enthalpy) and then dissolves in water (enthalpy of solution)
How is Hess' Law used to measure enthalpy change of solution?

Enthalpy of solution = lattice dissociation enthalpy + enthalpy of hydration
it can also be caluclated from lattice formation enthalpy but instead it’s
Enthalpy of solution = enthalpy of hydration - lattice formation enthalpy
Is enthalpy change of solution endo/exothermic?
Depends on lattice enthalpy and enthalpies of hydration
When is enthalpy change of solution endothermic?
When lattice enthalpy of formation is more exothermic than enthalpies of hydration
When is enthalpy change of solution exothermic?
When enthalpies of hydration are more exothermic than lattice formation enthalpy
Is enthalpy change of hydration endo/exothermic and why?
Exothermic - water molecules are attracted to ions, releasing energy
What factors affect enthalpy of hydration?
Ionic size/radius and ionic charge
How does ionic radius affect enthalpy of hydration?
Smaller ionic radius makes enthalpy of hydration more exothermic
Smaller ionic radius means greater charge denisty so greater attraction between ions and water molecules
More energy is released when the bond is made
How does ionic charge affect enthalpy of hydration?
Greater ionic charge makes enthalpy of hydration more exothermic
Greater ionic charge means greater charge denisty so larger attraction between ions and water molecules
More energy is released when the bond is made
What must occur for a reaction to take place?
a collision in the correct orientation of molecules with a minimum amount of EK (the EA)
What is a Maxwell-Boltzmann distribution/curve?
A graph which shows the amount of energy each particle present in a chemical system could have. It is only for gas particles but the conculsions that we can draw from it can be applied to liquids as well.
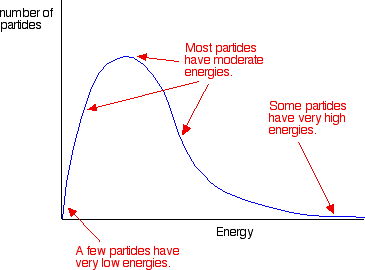
What is the general shape of a Maxwell-Boltzmann distribution?
What is the area under a Maxwell-Boltzmann distribution equal to?
total number of molecules
Why does the curve of a Maxwell-Boltzmann distribution start at 0?
no molecules have 0 energy
What does the peak of a Maxwell-Boltzmann distribution curve represent?
most likely energy of any single molecule
Where is the mean energy of all the molecules on a Maxwell-Boltzmann distribution curve?
a point slightly right to the peak/most likely energy
Where is the EA on a Maxwell-Boltzmann distribution curve?
(towards bottom of curve on RHS)
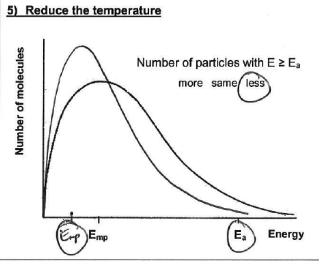
How will the shape of the Maxwell-Boltzmann distribution change if the temperature is increased? why?
curve shifts to right and peak lowers (area stays the same as total no. of molecules doesn’t change
because a greater proportion of molecules have at least the EA and are able to react
How will the shape of the Maxwell-Boltzmann distribution change if the temperature is decreased & why?
curve shifts left and peak gets higher (area stays the same as total no. of molecules is changed)
because a fewer proportion of molecules have at least the EA and are able to react
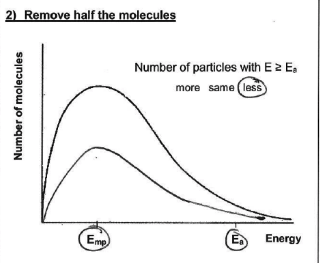
How will the shape of the Maxwell-Boltzmann distribution change if the concentration is increased? why?
peak gets higher - area increases
more molecules available to collide in a given volume and more molecules w/ energies > EA
How will the shape of the Maxwell-Boltzmann distribution change if the concentration is decreased? why?
peak gets lower - area decreases
fewer molecules available to collide in a given volume and fewer molecules w/ energies > EA
How will the Maxwell-Boltzmann distribution change w/ the addition of a catalyst and why?
no change in shape
but EA lowered so moves slightly left of curve
as more molecules have energies at or higher than EA
What are some techniques for measuring rate of reaction?
mass loss
gas production
colorimetry
What are the typical units of rate of reaction?
mol dm^-3 s^-1
What is the basic equation for a rate of reaction?
change in concentration/ change in time
What is a colorimeter or spectrometer?
measures the light intensity of light passing through a sample, intensity of light is measured every few seconds and data is plotted, light intensity is related to the concentration
How can you measure the rate of reaction using changes in mass?
when gas is produced in a reaction it escapes from the reaction vessel so the mass of the vessel decreases, can be measured using balance, the cotton wool in the neck of the flask allows gas to escape
What is a limitation of measuring loss in mass to measure the rate of reaction?
gas must be sufficiently dense or the change in mass is too small to measure on a 2 or 3 decimal balance
How can you measure rate of reaction using changes in volume of gas?
gas collection can involve collecting gas through water by displacement using a burette or inverted measuring cylinder, this method can only work if gas has a low water solubility
What does collision theory state?
for a reaction to occur:
particles must collide with enough kinetic energy
rate of chemical reaction depends on how often successful collisions happen
What is collision theory affected by?
how often particles collide
the energy each particle has when they collide
the minimum energy needed for reaction to occur: activation energy
collision geometry - orientation or angle when particles collide
What can collision frequency (how often particles collide) be altered by?
change in total pressure
change in concentration of reactants
change in temperature
change in surface area
How does pressure affect rate of reaction?
the same number of particles occupy a smaller volume, resulting in increased collision frequency
How do catalysts impact rate of reaction?
they lower activation energy so more particles will have sufficient energy to react
energy profile of exothermic reaction
energy of reactants are higher than products, change in products - reactants is less than 0
energy profile of endothermic reaction
products have higher energy than reactants and change in products - reactants is greater than 0, (positive 🔺H)
How does a catalyst impact the rate of reaction?
it provides an alternative pathway for a reaction to occur, lowering the activation energy
How does raising the volume of a reactant impact the rate of reaction?
it lowers the rate of reaction because the same amount of particles being in a larger space increases the distance between them, leading to lower frequency of successful collisions
What is order of reaction?
The index to which the concentration of a reactant is raised in the rate equation
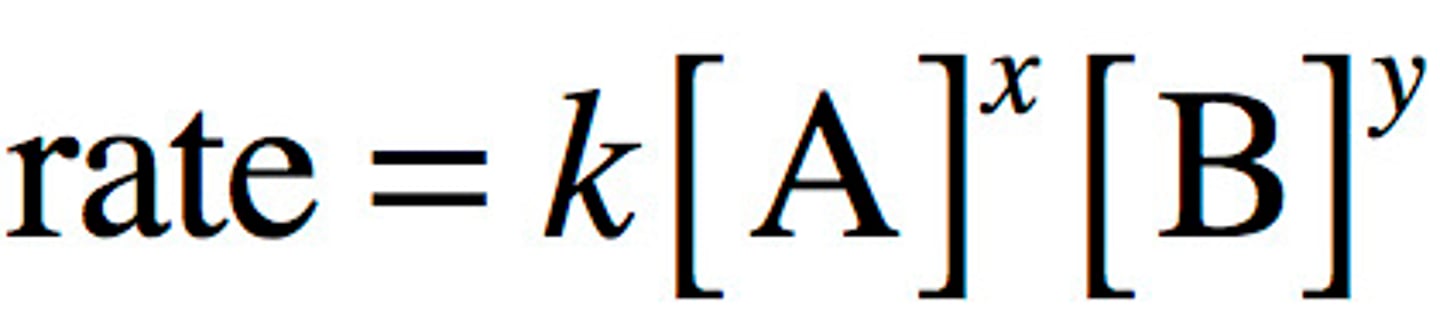
What does it mean when the concentration doubles and the rate doubles?
order 1
What does it mean when the concentration doubles and the rate stays the same?
order 0
What does it mean when the concentration doubles and the rate quadruples?
order 2
What is the rate constant?
Proportionality constant that converts between rate and concentrations and orders
What is a half-life?
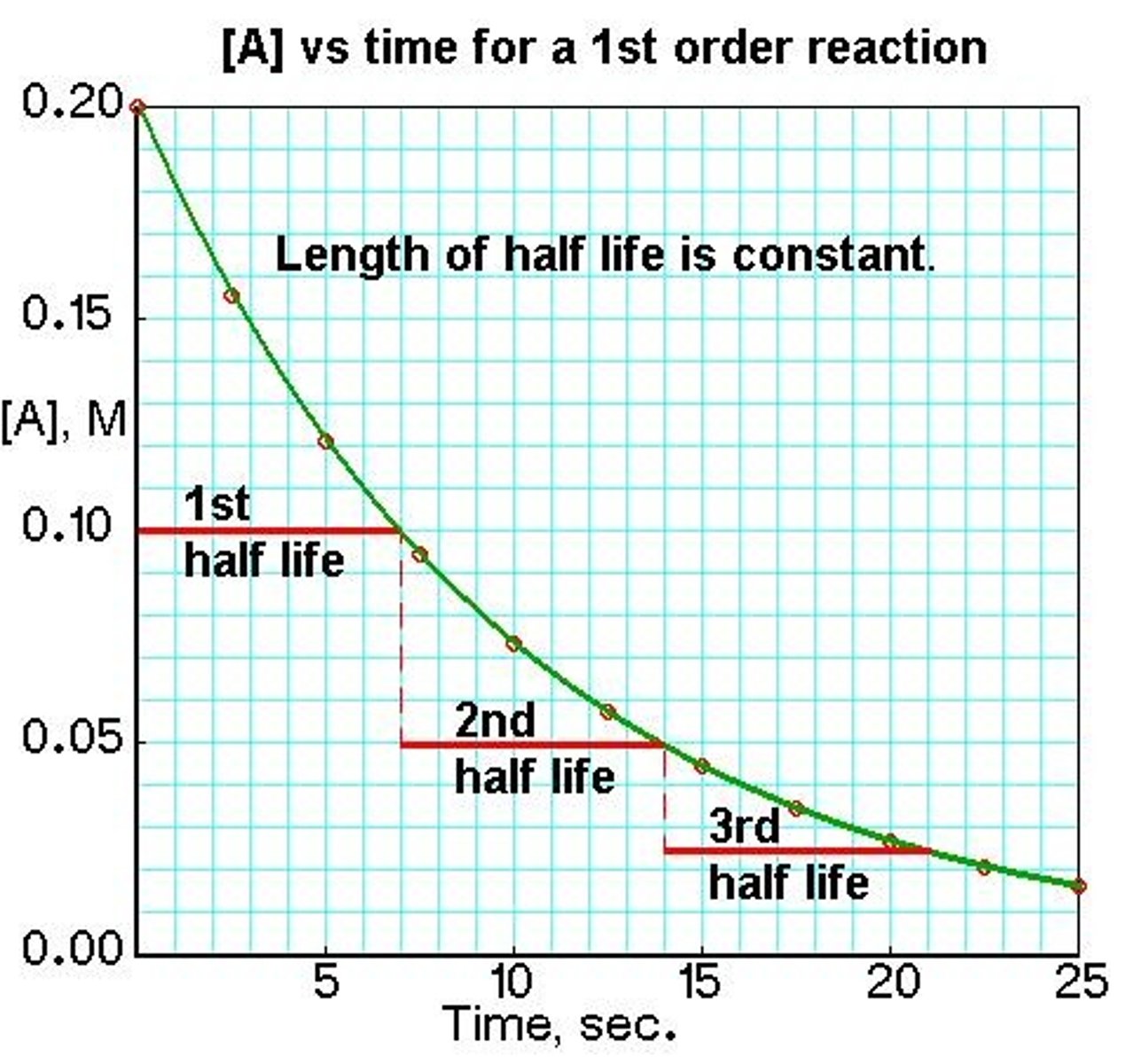
Time taken for the concentration of a reactant to fall to half of its original value
What is the rate determing step?
The slowest reaction in a multi-step reaction
What’s the relationship between the rate and concentration of reactant in a zero order reaction?
the rate is independent of the concentration of the reactant → same amount of concentration decomposes over time
What is the general form of the rate equation?
![<p>Rate = k[A]^m[B]^n</p>](https://knowt-user-attachments.s3.amazonaws.com/9d219407-8d95-4a07-911c-c93992012b4b.jpg)
What is the shape of a 0 order reaction on a concentration-time graph?
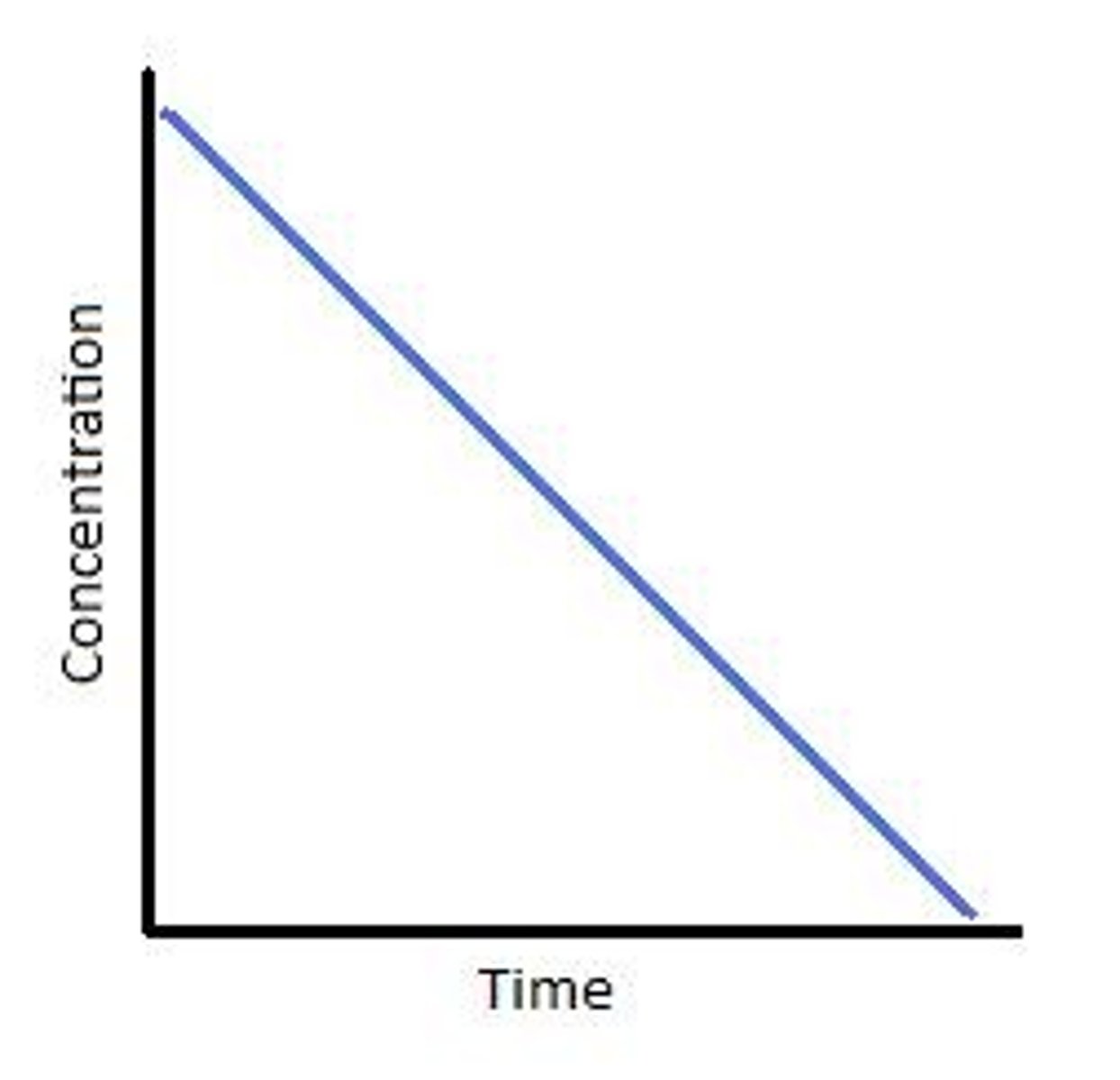
With gradient = k(rate constant).
What is the shape of a first order reaction on a concentration time graph?
What is the shape of a 0 order reaction on a rate-concentration graph?
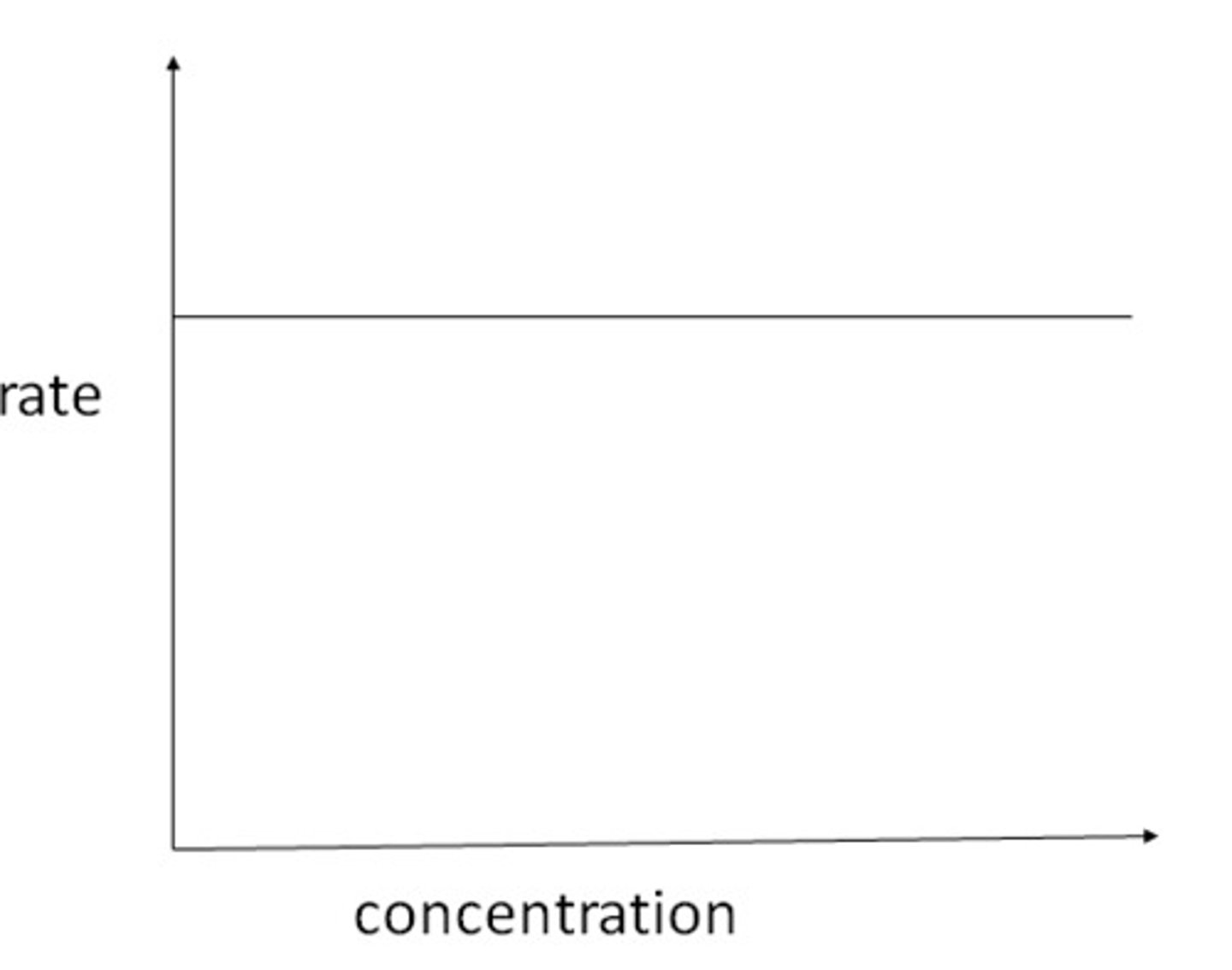
where the y intercept is k(rate constant)
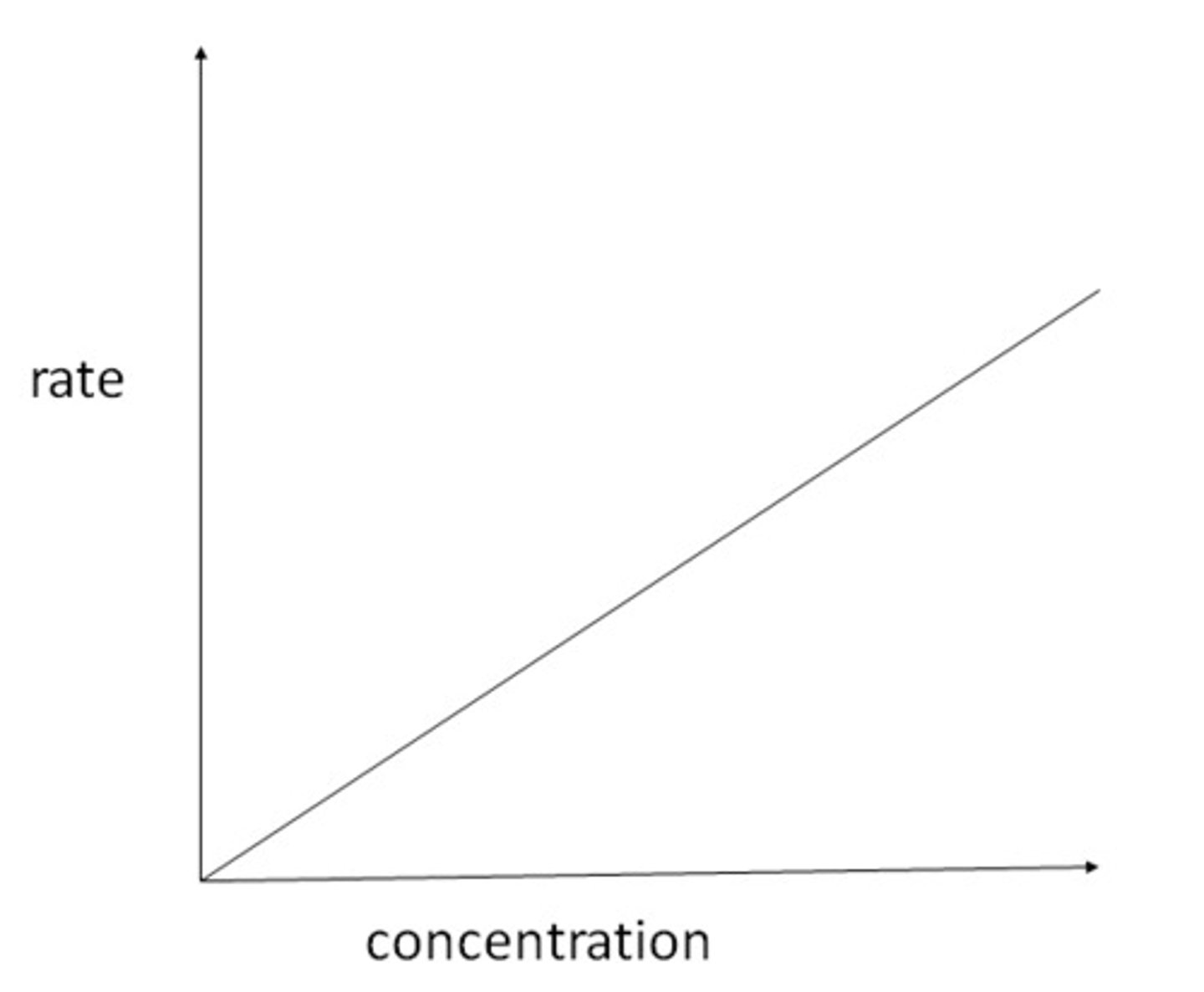
What is the shape of a first order reaction on a rate-concentration graph?
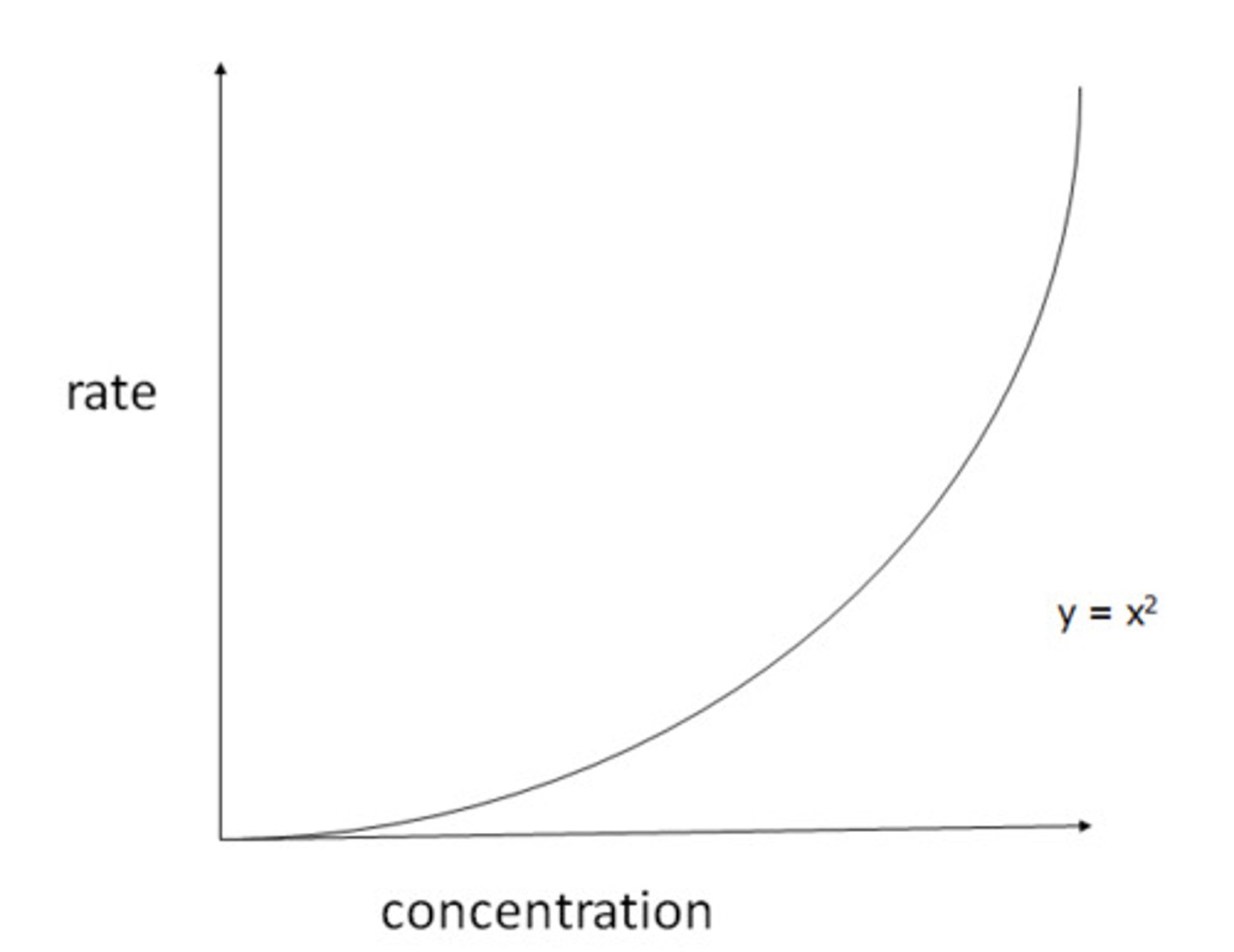
What is the shape of a second order reaction on a rate-concentration graph?
How can something which is zero order increase in conc but have no effect on rate?
For reaction : rate = k [C]1 [S]0 = k [C]1 x 1 = k [C]1
[S]omething can increase in concentration but have no effect on rate because the reaction between [C]1 & [S]0 has multiple steps like in organic mechanisms e.g. electrophilic subsitution etc.
This would mean there is an intermediate formed between Step 1 & Step 2 etc etc that is the one colliding etc and it is the rate of formation of this intermediate that effects the rate and not [S].
Why might there be multiple steps in a reaction where one substance is zero order?
It could be that the non-0 order chemical (C) undergoes a chemical change that has nothing to do with the 0 order one (S). It may even happen when C is in the beaker on its own! This can happen by the collision of a particle of C with a solvent molecule in which it is dissolved. This is a known process.
How could you explain & represent a reaction mechanism of rate = k [C]1 [S]0 = k [C]1 x 1 = k [C]1?
Now let’s say that C is being slowly converted into something new called π. Let's write an equation for this equilibrium process. Because this is an equilibrium process there will only be a limited amount of π at any time, especially if the equilibrium lies to the left:
STEP 1: C ⇌ π slow reaction
As soon as is π produced, it reacts with S in a very fast reaction to produce the product D. The equation is:
STEP 2: S + π ➔ D fast reaction
Overall, we add the equations together cancelling anything we can. We do this all the time in chemistry.
C ➔ π STEP 1(slow)
π + S ➔ D STEP 2 (fast)
C + S ➔ D Overall
If you wanted to include a solvent molecule, sol, in the process, you could write it as follows
C + sol ➔ π STEP 1(slow)
π + S ➔ D + sol STEP 2 (fast)
C + S ➔ D Overall (the solvent molecule was regenerated in step 2)
Why in multiple step reaction mechanisms can we not observe the reactive intermediate π?
This is because it doesn’t hang around. As soon as it is made, it is consumed very quickly by S.
If we looked hard enough, we might be able to capture some .
What more detailed, analgous explanation as to why something which is zero order increase in conc but have no effect on rate?
If the “reaction” is rate of dirty dishes produced by a bakery then our “reactants” will be the number of bakers and the number of customers.
If the number of customers increases, the rate of dirty dishes produced won’t increase.
This is because the “hidden intermediate” is number of pasteries baked since if the bakers can only make 2 pasteries only 2 dirty dishes will be produced even for a crowd of 1000.
This means only when number of bakers and so number of pasteries increases does rate of dirty dishes.
What can you assume for reaction mechanisms?
If any overall reaction has a chemical involved which is found to be zero order, you should assume that it must be a multi-step reaction, i.e. 2 or more steps.
At A Level, you should also assume that the first step in a multi-step reaction is the rate determining step.
How can you write a reaction mechanism from a rate equations/ orders of reactants e.g. NO2(g) + CO(g) ➔ NO(g) + CO2(g) with rate = k [NO2]2?
We have to make assumptions about the reaction and if at the end of the mechanism we get the reaction equation we have done it right
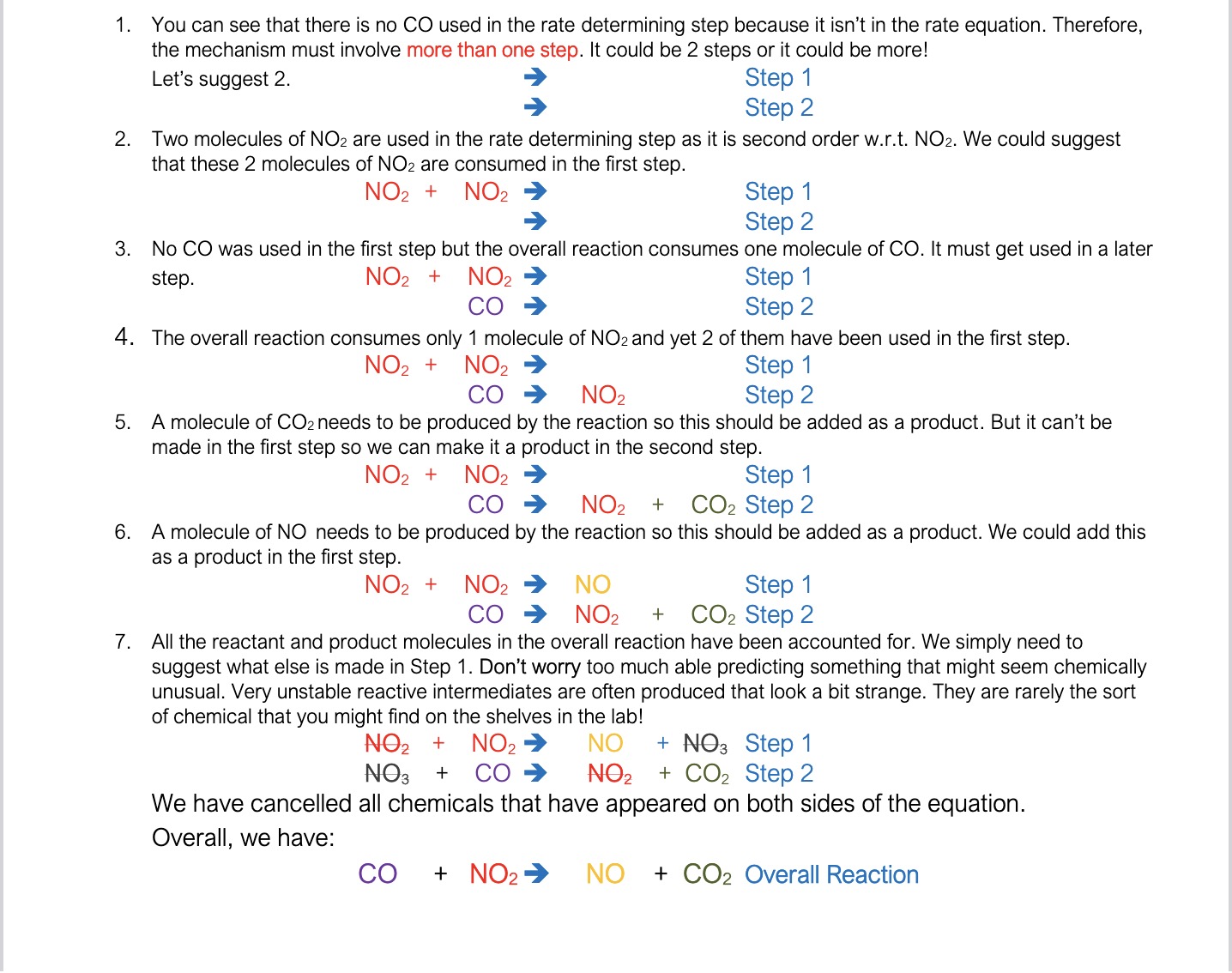
What are some “rules”/common assumptions to make when writing a reaction mechanism from orders of reactants etc?
If there is a chemical in the overall equation that is not in the rate equation, then it can’t be in the rate determining step. You should therefore assume that the reaction takes place in at least 2 steps.
If there is only molecule of a particular reactant in the overall reaction but it is second order w.r.t. to that reactant, then you know that you must use up 2 molecules in rate determining step but you must regenerate one molecule of the ‘reactant’ in a later step.
If there are 2 molecules of a particular reactant in the overall reaction but it is first order w.r.t. to that reactant, then you know that you must use up 1 molecule in rate determining step, but another reactant molecule must be consumed in a later step.
You can generally assume at A level that all orders are whole numbers. (Many reactions have non-integer orders but don’t worry about that at A level. I’m just being honest!)
You can generally assume that the rate determining step is the first step
What is a clock reaction?
Measuring the time from the start of the reaction to a visual change occurring
What is the continuous monitoring method?
Taking constant measurements throughout the duration of the reaction
What is the assumption made in clock reactions & the inital rate method?
Taking constant measurements throughout the duration of the reaction
What are Lowry-Brønsted acids & alkalis?
Lowry-Brønsted acids donate protons in the presence of a base
Lowry-Brønsted bases accept protons in the presence of an acid
*an acid or a base cannot act as an acid or base unless its “prompted” to do so by being reacted with a base or acid
What ion causes a solution to become acidic?
H+/ protons but actually/in practice H3O+/hyronium ions- H+ reacts with H2O to form it.
However you can usually still write acids as releasing H+ ions/protons.
What ion causes a solution to become alkaline?
-OH - hydroxide ion
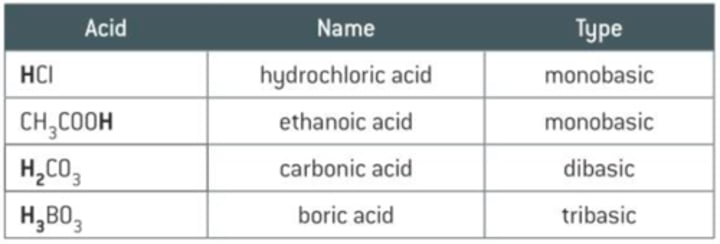
What are mono/di/triprotic acids?
Monoprotic acids dissociate One H⁺ in solution.
Diprotic acids dissociate Two H⁺'s in solution.
Triprotic acids dissociate Three H⁺'s in solution.
How do you calculate pH?
pH = -log[H⁺]
Why is a pH scale useful compared to concentration of H+?
pH scale allows a wide range of H + concentration to be expressed as simple positive values.
If two solutions have a pH difference of 1, what is the difference in [H+]?
A difference of 10x - pH is a logarithmic scale.
How do you calculate the pH of a strong acid?
For strong acids we can assume that 100% of the HA will dissociate into H+
So the concentration of HA = concentration of H+ formed
pH = -log[H⁺]
However if the acid is diprotic or triprotic we need to double or triple the concentration of HA