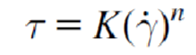Processing Prowess
1/196
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
197 Terms
Processing cycle
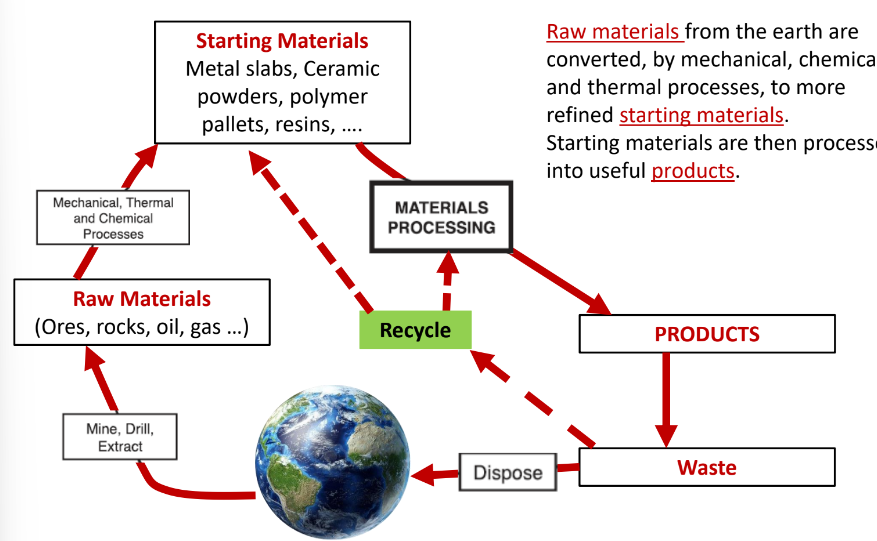
Definition of Materials Processing
Materials processing is the series of steps that converts as starting materials into functional form with controlled structure and properties.
Three approaches to Material Processing
1) Forming
2) Additive
3) Subtractive
Melt Process
A melt is poured or forced into a mold, through a die, or onto a surface. As it cools, it solidifies into the shape. The melt can also be shaped further before it solidifies. Results in constant cross section
Solid Processes
formation of 3D shapes or constant cross-section shapes by plastically deforming a solid using mechanical stress (e.g., with a die or roll), or by shaping solids through subtractive machining processes.
Powder Processes
Form 3D shapes by filling a die or mold with powder and applying uniaxial or isostatic pressure, optionally with heating. Post-processing such as sintering is typically required for densification, and many additive processes also use powders.
Dispersion and Solution Processes
Forming 3D shapes, sheets, or coatings from a dispersion of particles in a liquid or a polymer solution. Solidification usually occurs as the liquid is removed, such as by drying.
Vapor Processes
transforming vapor into a solid using methods like evaporation, sputtering, or chemical vapor deposition. Subtractive processes then define 2D regions and, with repetition, build 3D structures.
Do we typically change the composition of starting materials
No, only major expectations is manufacturing thermosets
Common Metal Processing steps (non-powder)
1) Extraction of Metal through ore
2) Formulated composition into alloys
3) Melt and cast into standard shapes
4) Solid deformation operations into smaller shapes
5) machining in 3D shape + post-processing
Common Metal Powder Processing steps
1) Extraction of Metal through ore
2) Make into powder
3) compacted into a shape or added in 2D layer
4) Sintering
5) machining in 3D shape + post-processing
Advanced ceramics
aluminum oxide (alumina, Al2O3), silicon dioxide (GLASS, silica, SiO2 ), silicon nitride (Si3N4)
Traditional ceramics
composed of clay materials
Common Ceramic process (non glass)
1) ceramic powders extracted or chemically synthesized
2) Compacted into shape or suspended and molded
3) Sintering
4) Post Process Thermal treatment
Common glass process
1) ceramic powders and minerals
2) Melting
3)Molding + Blowing or Flowing
4) Post process thermal treatment
Common thermoplastic process
1) Get pellets, particles, etc
2) Extrusions or molding
3) Cutting or machining
Common thermoset process
1) Monomers and curing agents (resins)
2) Flow resin and curing agents into mold
3) Solidify in mold and cool
4) machining
What characteristics do the starting materials affect?
1) properties and performance of final product
2) The processing steps
How to read steel metal grades
1st number shows the class of steel (2000 for nickel steels)
2nd number is a significant composition
3rd and 4th are C wt% *100

Why are most metals starting materials originate from ores?
It is thermodynamically stable for most metals to oxidize
Extractive Metallurgy
Processes to remove metal from ore, always involves crushing and separating first
Pyrometallurgy
uses heat to reduce metal compounds in ores with rxn with carbon at high temps, with strict control over temperature and atmosphere. Iron is example
Hydrometallurgy
extracting metals from aqueous solutions by leaching, purifying, and depositing the metal, often using ion replacement or bubbling hydrogen reduction at low temperatures. Example: Copper is extracted by adding iron to a copper sulfate solution and Cu precipitates out
Ion replacement
one ion in a solution is replaced by another ion to precipitate out as a pure metal
Bubbling Hydrogen reduction
Hydrogen gas is bubbled through a metal ion solution, reducing the ions to solid metal, which then precipitates out.
Electrometallurgy
use electrical energy to convert metallic ions into metals. Ions are leached from ore in aqueous solution or molten salt, then electrolysis is used to form the metal. Aluminum is an example
How is scrap metal used in metal processing?
Scrap metal is added at various stages of metal production. It offers economic and environmental benefits. Controlling its composition can be challenging.
2 most common form of iron ore
magnetite (Fe2O4) and hematite (Fe2O3)
Pig Iron Production
1) Add Coke, Iron Ore, and Limestone to the blast furnace
2)coke reacts to O2 to generate CO and heat
3) CO reduces the ore which completes at 1600 C.
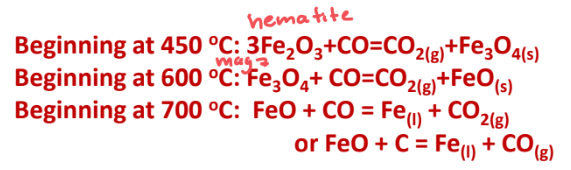
How is coke made
Coal undergoes pyrolysis (heating without oxygen)
Role of Limestone
Acts as a flux, decomposes to CaCO3 which reacts and removes SiO2 from iron melt and forms slag

Basic Oxygen Furnace (BOF)
1) Pure O2 blown into molten pig iron, scrap (~30% )and flux
2)O2 reacts with C to lower C content and other impurities to make slag
3) slag is removed
4) Excess Oxygen will need to be removed later
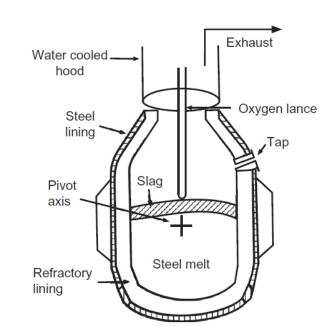
Electric Arc Furnace (EAF)
1) Graphite electrodes melt pig iron and scrap by passing a large current through the charge
2) O2 is added to oxidize impurities
3) Lime flux is added near the end
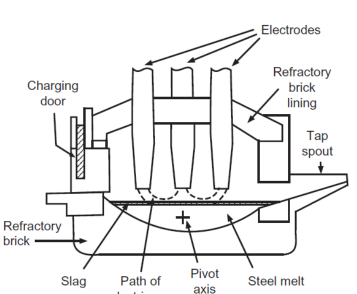
Which is better EAF or BOF
EAF because it can use more scrap which is cheaper and better for environment
Ladle Metallurgy
1)Remove from furnace into ladle
2) Refine chem by adding deoxidants to remove O2 and make slag
3)Remove slag and ingot or continuously cast
Overall Steel Process
1) Make pig Iron from iron ore and coke at 1600 C
2) Use BOF or EAF to remove impurities with O2
3) Use Lime and other fluxes to remove impurities in slag
4) Pour into ladle and remove excess O2
5) Cast into ingots or continuously cast into smaller shapes
Main source of Al
Bauxite
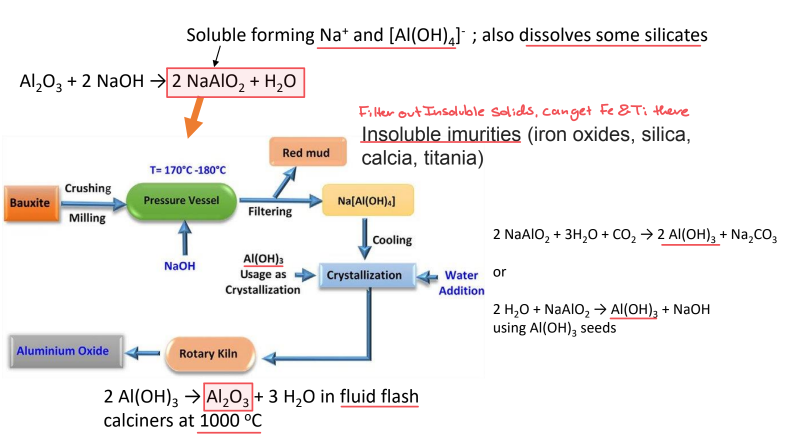
Bayer Process
1) Crush Bauxite
2) Add NaOH to dissolve Al2O3 into NaAlO2
3)FIlter out insoluble impurities
4)Cool and added with Al(OH)3 crystals to precipitate aluminum hydroxide (Al(OH)₃) from the solution.
5) Heat Precipitates to remove water and make alumina (Al2O3)
Hall-Heroult Process
Continuous Process
1) Alumina dissolved in dissolved in a mixture of cryolite (Na3AlF6), aluminum fluoride (AlF3), and a little fluorspar (CaF2) at about 940 C.
2) Carbon Anodes reduce the Al ions to metal Al and some CO and more CO2 are released
3) Liquid Al is dense and collects at the bottom to be removed
Why is recycling Al important
Hall-Heroult Process is energy intensive and releases lots of CO2
Estimating size of irregular paticle
1)equated to the diameter of a sphere that has the same volume
2) the diameter of a sphere that has the same projected area
Sieving
Weight Distribution, Powder is passed through sieves with progressively smaller openings. The powder retained on each sieve is weighed to determine the PDI
Microscopy
Number distribution, Size and shaped observed, but time consuming
Sedimentation by X-ray absorption
Weight distribution, Sedimentation rate under gravity varies with particle size. X-ray adsorption measures particle concentration at specific locations over time
Laser Difraction
Volume distribution, Laser light diffraction from particles in a liquid is measured to determine size. The spatial distribution of diffracted intensity is proportional to size
Photon Correlation Spectroscopy
Number distribution. Suspension of particles in a liquid is light with coherent light, and the scattered light is measured. The Brownian motion of small particles modulates the scattered intensity, which is related to particle size.
List of Metal Powder Processes
Mechanical Methods
Melt Atomization
Oxide Reduction
Chem and electrochem processes
Powder processing with Mech Methods
Can be made from chips and shavings, but hard to do with ductile materials
Melt Atomization
A stream of liquid metal is broken up (atomized) by gas or water jet into droplets that solidify into particles as they cool.
Oxide Reduction
High-purity, size-controlled metal powders are made by heating ceramic powders in a reducing atmosphere to convert them into metal powders.
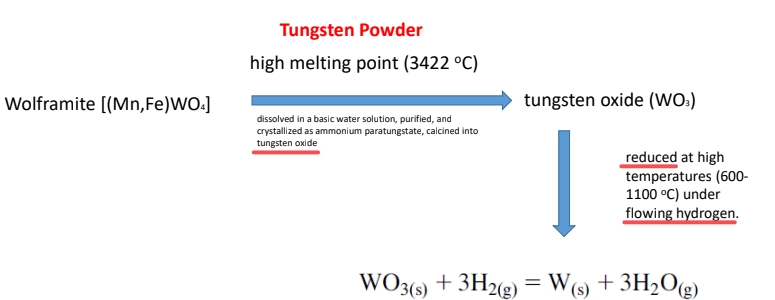
Chemical and Electrochemical Processes
Metal powders may be formed by precipitation due to chemical or electrochemical reduction of aqueous salt solutions.
Ways to get alloyed powders
Prealloyed powder
Admixed powders
Bonded powder
How to tailor PDI
Sieve
Blend - add 2 diff sizes
Comminution - Mill
Additives - Add binders etc
Get average diameter size
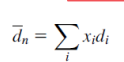
4 ways to make ceramic powders
Mineral Process
Chemical Solution Process
Solid State Reaction Process
Gas Phase Process
Ceramic Powder Mineral Process
Mined materials like clay and quartz are crushed and processed to remove impurities and standardize particle size. Advanced ceramics, such as zirconia and alumina, undergo further purification and processing
Powder Chemical Solution Processes
Inorganic chemicals are dissolved in water or organic solvent to produce metal oxides thru precipitation, gelation, or evaporation. Then heated to produce final oxide powder
Ceramic Powder Solid State Reaction
Multicomponent ceramic oxide compositions can be prepared by solid state reaction of materials created by mineral-based or chemical solution processes.
Ceramic Powder Gas Phase Process
Gases or vapors as one or more of the reactants. Metal Chloride vapor and water vapor heated in a reactor or passing gas over solid reactant.
Comminution
Reducing particle size and break agglomerates thru crushing,millimng and grinding
Bigger parilces break up first due to more flaws
Ceramic Additives
- Binders (to bind particles to each other)
- Dispersants (to prevent particles from clumping together and dispersed
- Surfactants (reduces surface tension)
- Deflocculants (to scattered particles in suspension)
Glass starting material
Glass formers: SiO2, B2O3
Flux: salt cake (Na2SO4) to lower melting point and viscosity
Other glass modifiers: limestone (CaCO3) for chemical stability and durability (positive charged modifiers)
Glass process
A continuous process to final product
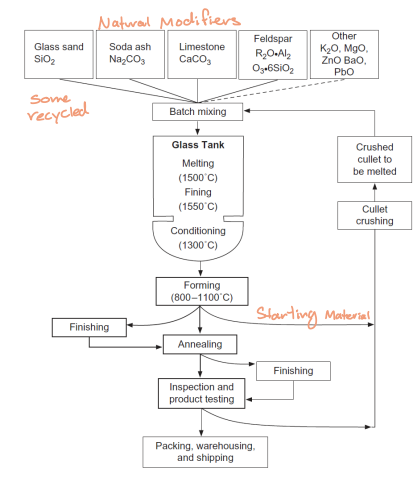
Why is viscosity of glasses important
Determines how easily the glass can be made, modifiers are added to dcrese viscosity
Chain growth polymerization
involves three steps: initiation, propagation, and termination
Step growth polymerization
involves the reactions of two chemically different monomers
The two species are typically difunctional (i.e., they can react at both ends) so that chains grow as reaction proceeds.
Number Average and Weight Average Formula
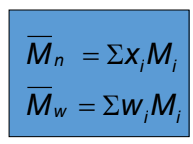
Average molecular weight formula
Thermoplastic starting materials
pellets, granules, flakes and powders
Thermoset starting materials
mixes that contain prepolymers or oligomers (low molecular weight polymers) as well as hardeners, curing agents, or initiators.
Bulk Polymeriztion
Reactants are combined with initiators in a controlled vessel to synthesize polymers, mainly through chain addition reactions. The process yields high-purity polymers but poses challenges in controlling temperature, molecular weight, and managing increasing viscosity during mixing.
Solution Polymerization
Solvent is used to dissipate heat during polymerization, with all components dissolved in the solvent. This method has a lower yield than bulk polymerization and requires solvent removal afterward.
Suspension Polymerization
A liquid is used to remove reaction heat, with reactants suspended as droplets. Additives maintain small droplet sizes, resulting in small polymer beads that are later formed into larger pellets. However, purity is lower due to the use of these additives.
Emulsion Polymerization
Water is used as a medium for monomer droplets and initiator in suspension polymerization. Surfactants stabilize the droplets and form micelles. The monomer diffuses into micelles and reacts with a water-soluble initiator, forming solid polymer particles suspended in water, known as latex, used in paints and adhesives.
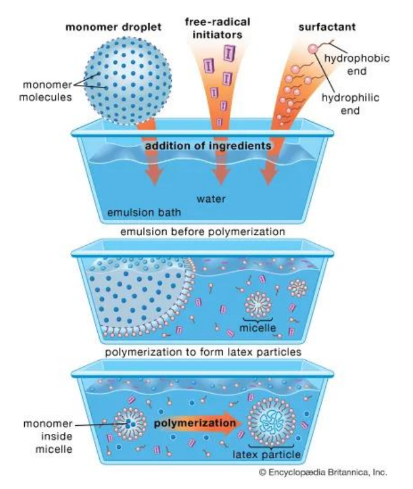
How to make Low density PE (LDPE)
High pressure bulk polymerization, high temp and pressure are used to increase the MW. Also lots of initiator is used to create many small chains which lowers density b/c it cant crystalize
Gas phase fluidize bed process
Makes both HDPE and LLDPE. Ethylene gas is fed into a reactor packed with catalyst and polymer particles to create reactive sites and polymerizes
DIfferent stages of resins
Stage A resins - no crosslinking
Stage B resins - partly polymerized
Stage C resins - fully crosslinked
Phenolics
(a) The reaction between phenol and formaldehyde to form a hydroxymethyl substituted intermediate
(b) condensation reaction between phenol and hydroxymethyl substituted intermediate to make phenol
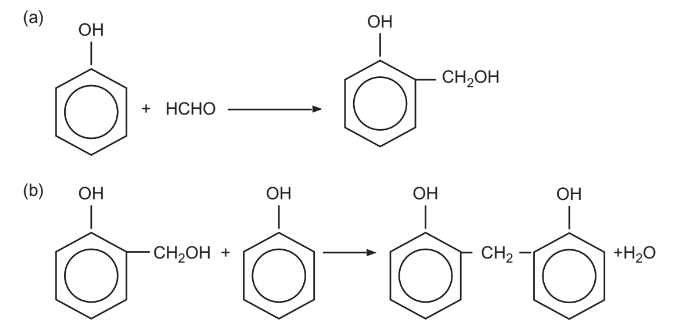
Resoles
One step
Liquid or solid resins made from phenol and formaldehyde oligomers (B stage).
Capable of reacting without additional additives.
Produced in small quantities with a limited shelf life.
Novolacs
Formed under conditions that produce low molecular weight polymers without reactive hydroxymethyl groups.
Water by-product is continuously removed.
Cannot form crosslinked polymers without a curing agent (2 step)
Have an infinite shelf life
3 steps in every melt process
Flow
Shape definition
Shape retention
Materials that use melt processes
Metals - most common method
Glasses
Thermoplastics
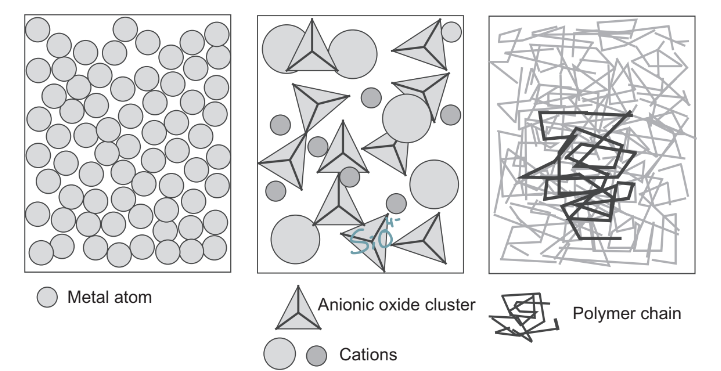
Models of melts for each material class
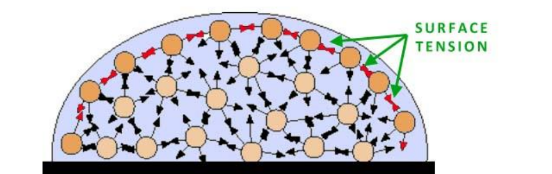
WHat causes surface tension
Surface molecules are more strongly attracted to their neighboring molecules in the liquid due to fewer molecules in the adjacent vapor phase.
This imbalance in forces at the liquid's surface results in surface tension.
Equivalent to surface energy/interfacial energy
Forms spherical shape to minimize energy
Do molten metals have the higher surface tensions
Yes because they have have stronger bonds (shon by Higher Tm)
Relation between Surface tension and bond strength
Stronger bonds lead to higher surface tensions
Contact angle
The contact angle is the angle formed at the interface between a liquid droplet and a solid surface.
It indicates the wettability of the surface: smaller angles represent better wetting, while larger angles indicate poor wetting.
What usually happens to surface tension as temp increases
Usually decreases (weaken bonds)
Do larger or smaller bubbles have a larger internal pressure
Smaller
Laminar Flow
Sliding of layers with infinitesimal thickness relative to adjutant layer, no mixing
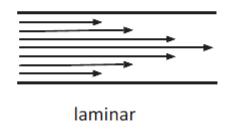
Turbulent flow
Irregular motion with fluctuation in velocity, promotes mixing
Fundamental difference between solid and liquid
Ability to withstand a shear stress, liquids can not
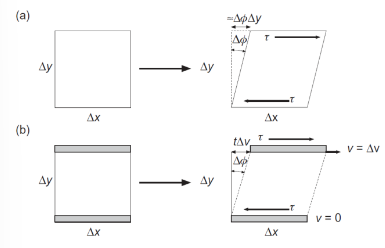
Shear strain rate
Can use velocity because top plate is moving with speed v while the bottom plate is stationary
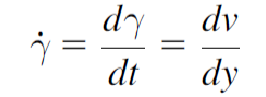
Viscous heating
Viscous heating occurs when a liquid is sheared, increasing its internal energy due to work done on it.
If no heat exchange with the surroundings occurs, the liquid’s temperature rises due to friction
Viscosity
Viscosity is a measure of a fluid's resistance to flow and deformation.
It quantifies internal friction within the fluid, determining how easily it can move.
Relationship between shear stress and strain rate (newtonian)
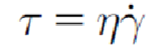
Shear thinning
is when a fluid's viscosity decreases with increasing shear rate.
This means the fluid flows more easily when subjected to higher stress or agitation
Viscosity’s relationship with temperature
Decreases with increasing temp
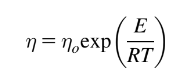
Non-Newtonian Fluid
fluid whose viscosity changes with the applied shear rate or shear stress.
Equation for non-Newtonian fluids