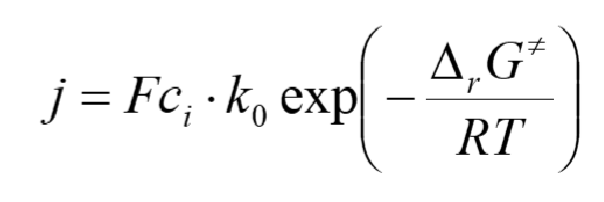Electrochemistry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What is Faradays Law of electrolysis
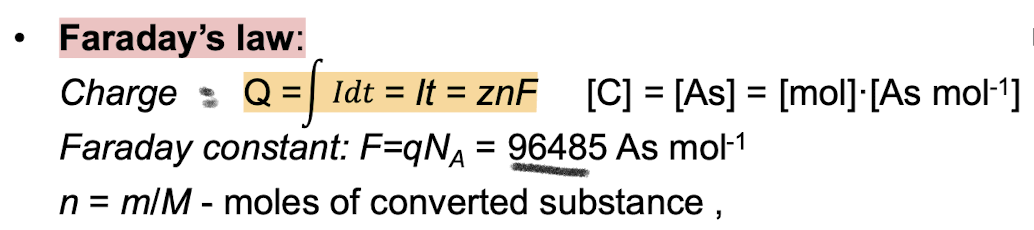
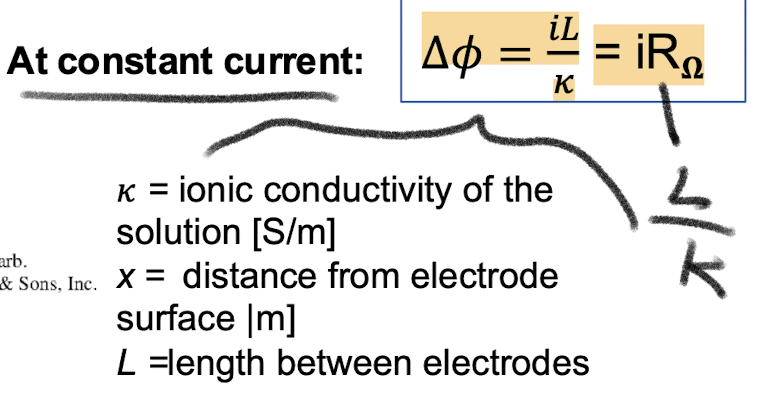
What is Ohms law
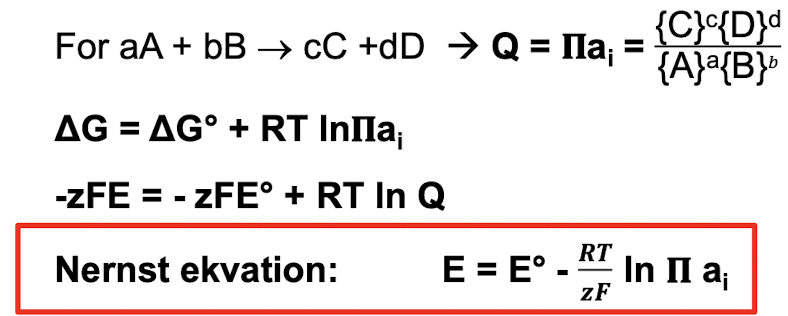
How do you calculate the energy needed for a reaction at standard conditions and non standard conditions
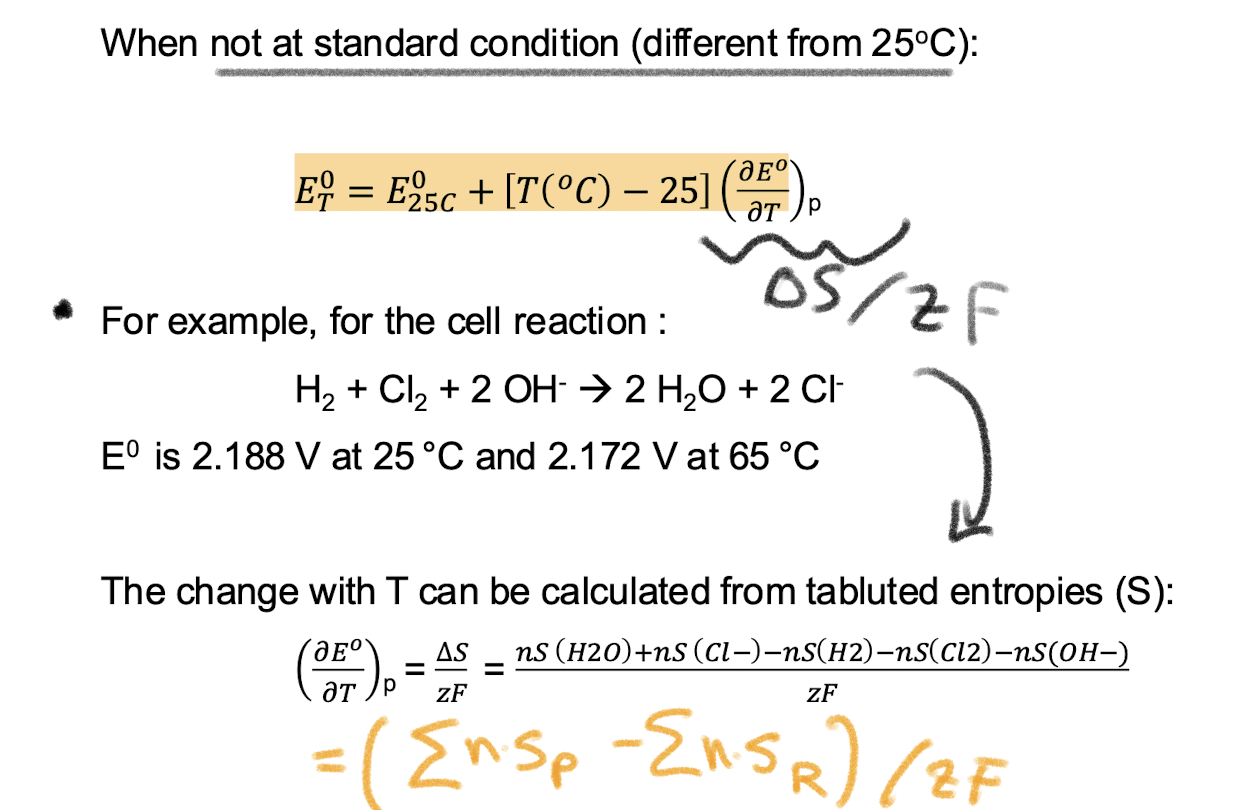
How do you calculate the potential of a reaction at non standard temperature
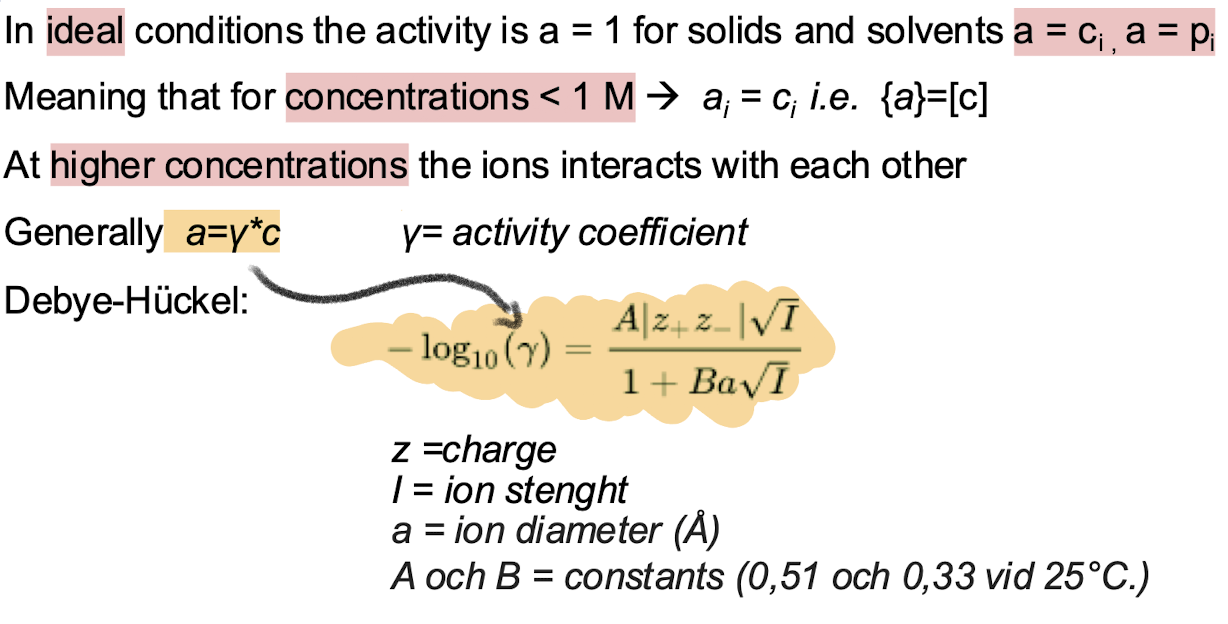
How do you relate the activity to the concentration
What is the interplay between the potential and the energy
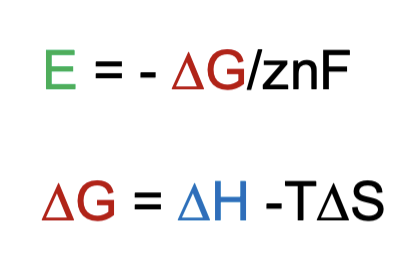
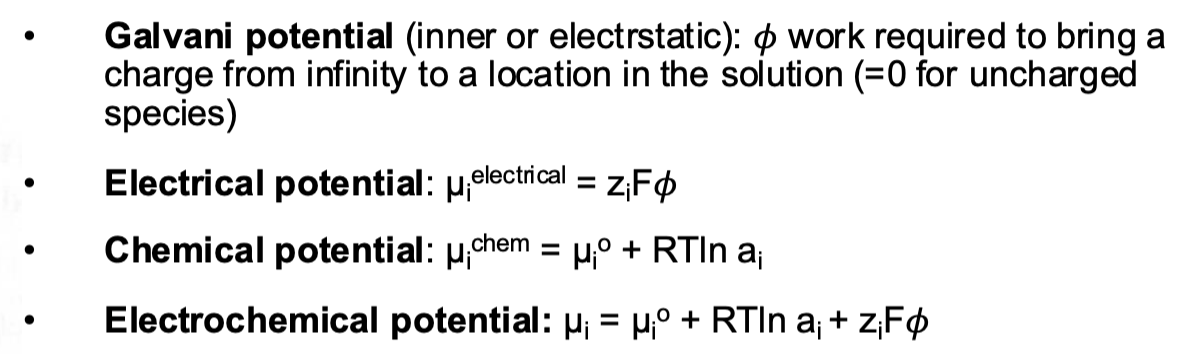
What different types of potentials are there
What are some common reference electrodes
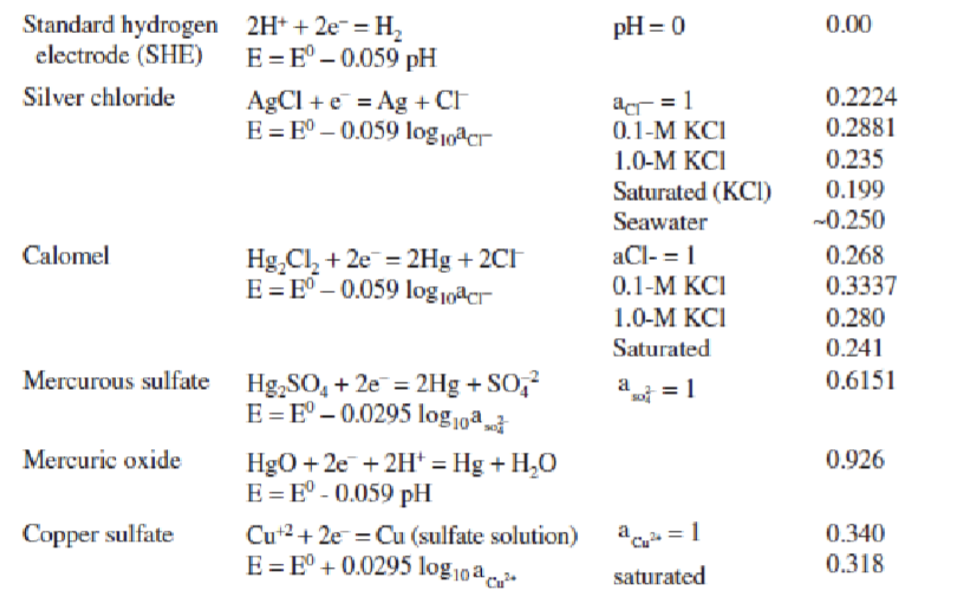
What is the electrochemical double layer
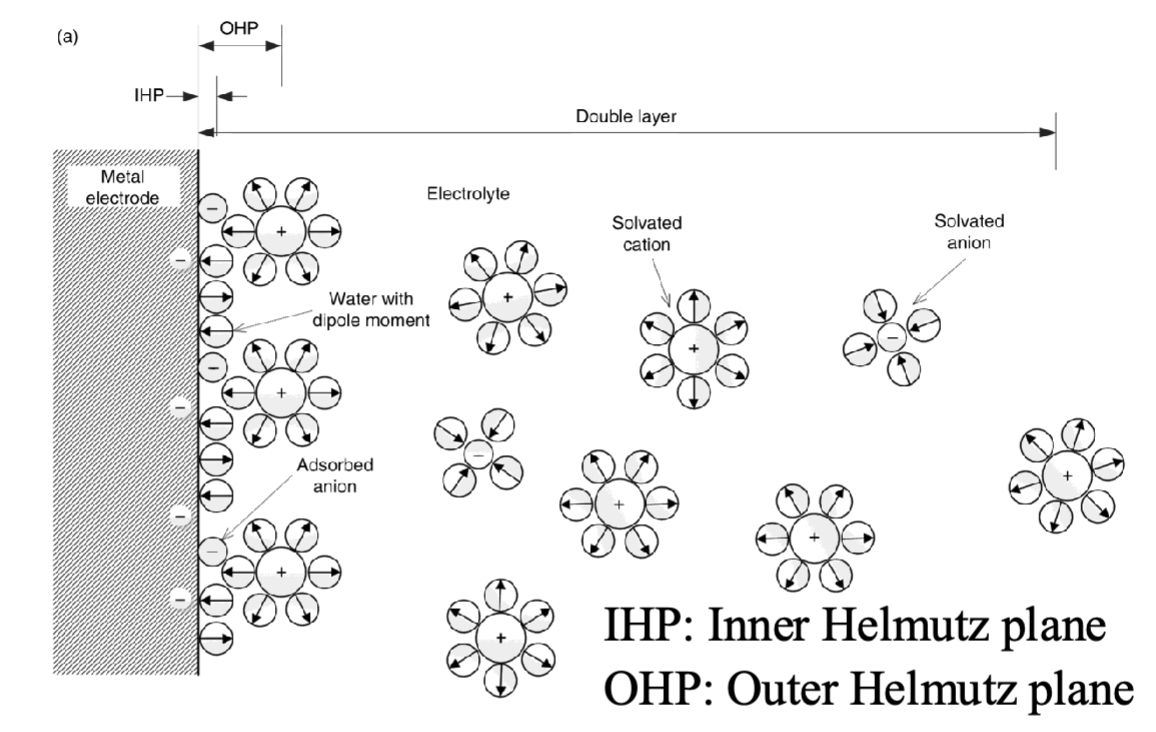
What different models for the double layer is there
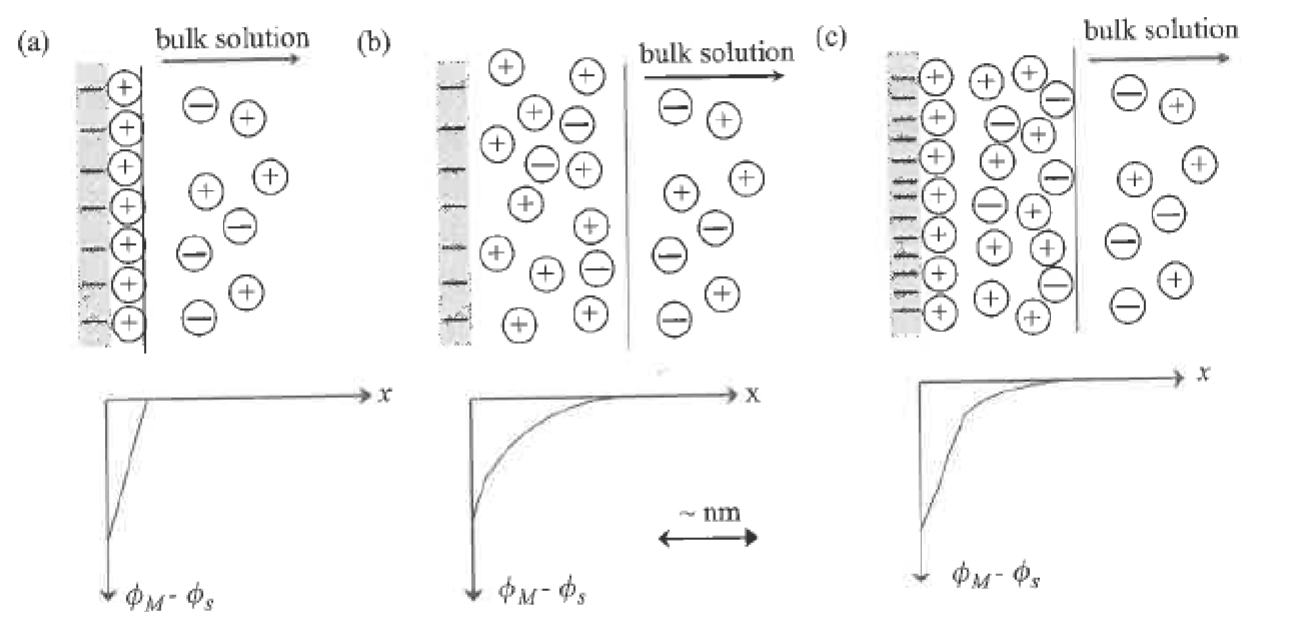
What differs in the potential drop when current is applied
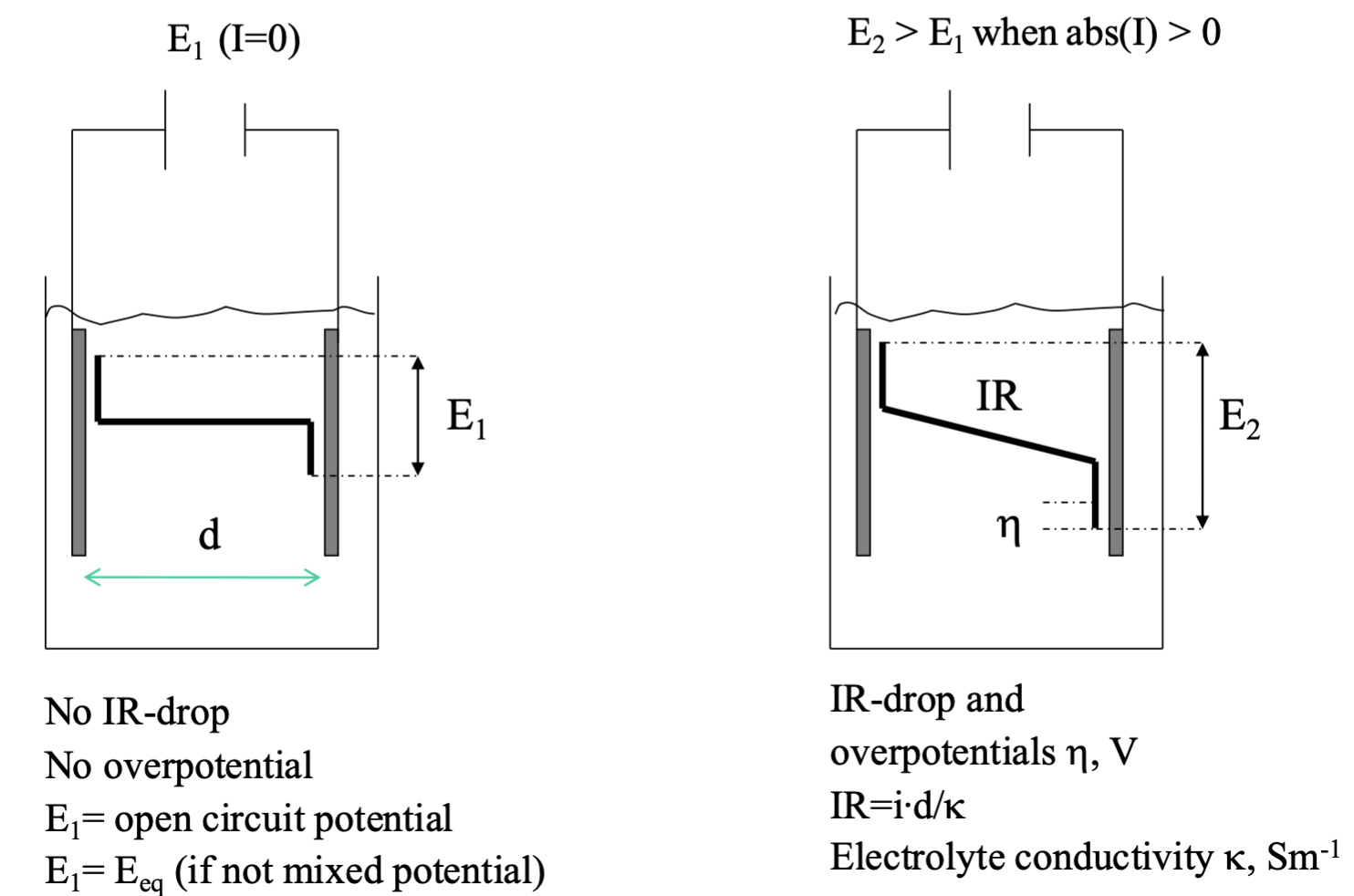
How does the overpotential differ at anode resp cathode for galvanic resp electrolysis
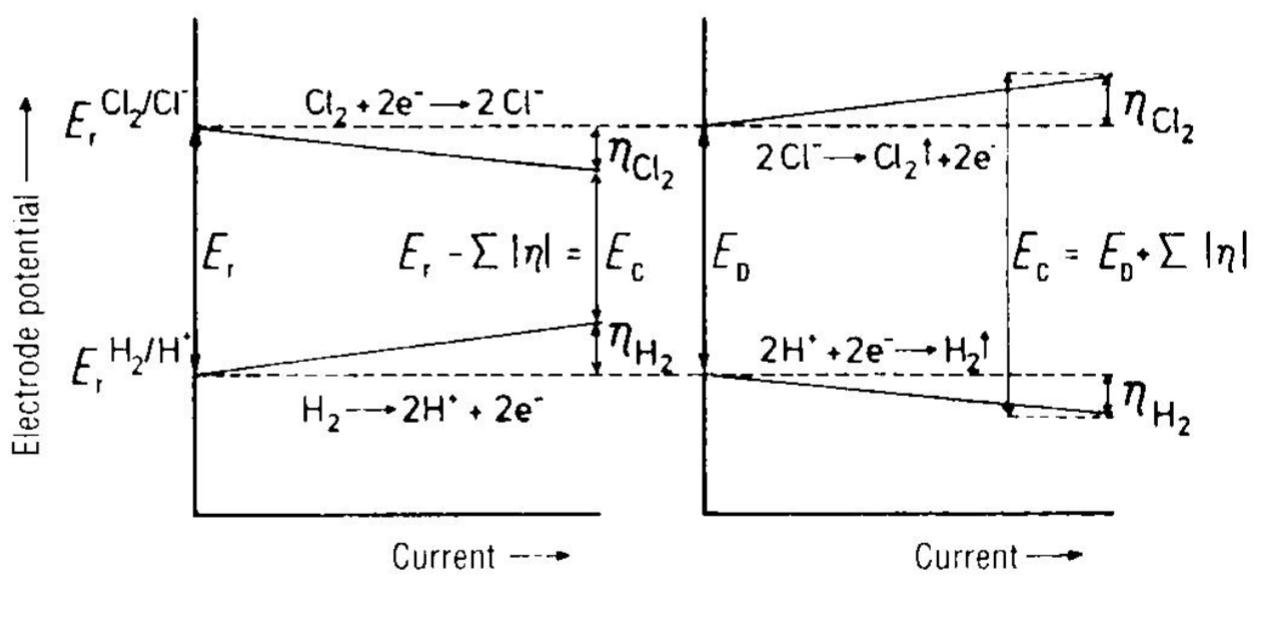
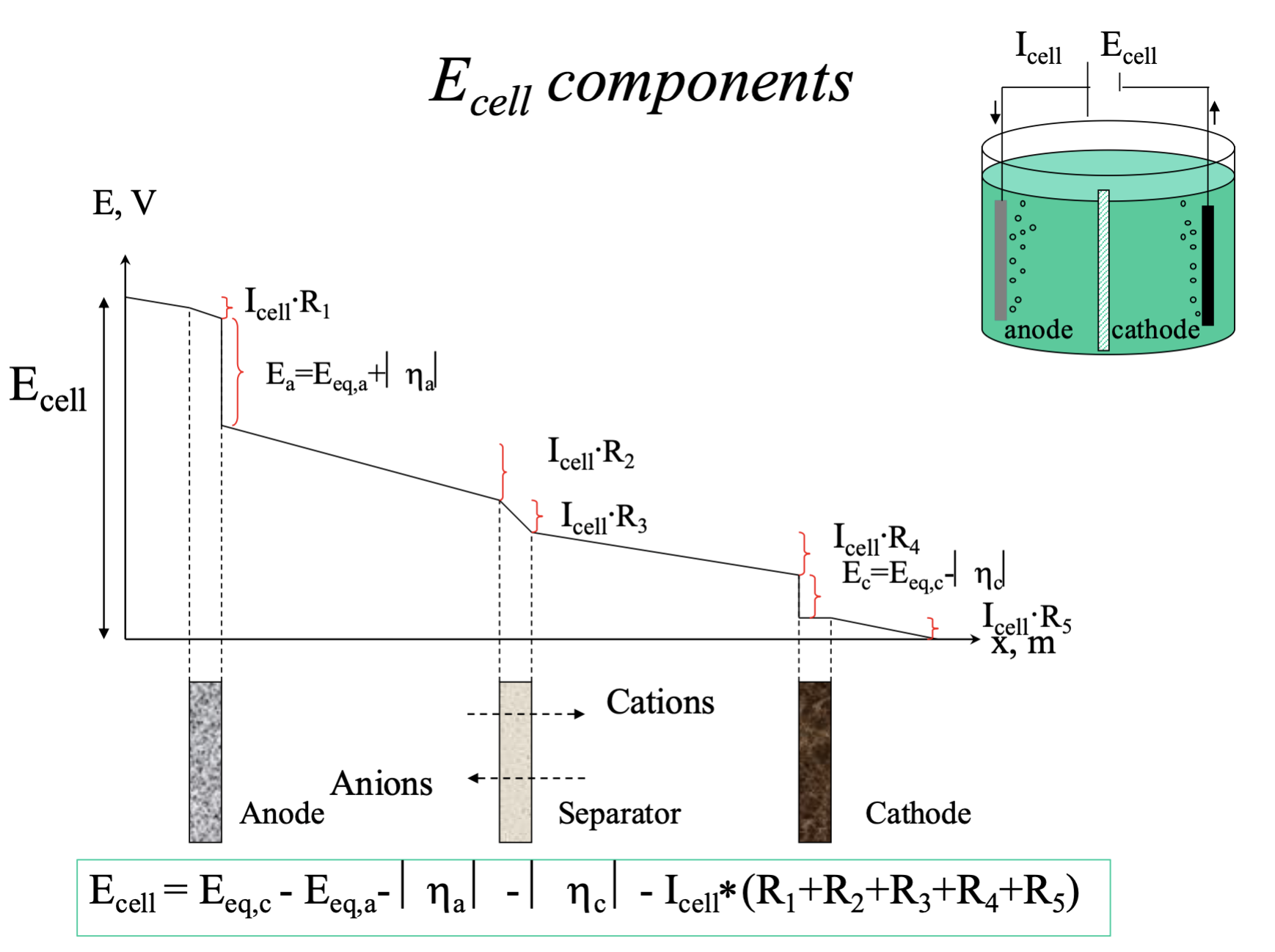
What different types of losses are there in a cell, why and where do they occur
What is the reaction rate for a reaction in flux and current density