4.5.1.3 The energy change of reactions
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Last updated 9:39 AM on 4/2/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
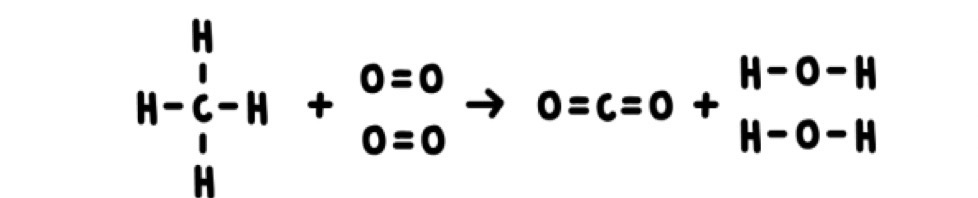
Each line represents a bond, two lines represent a double bond e.g. in the oxygen molecule.
2
New cards
During a chemical reaction what is needed
* Energy must be supplied to break bonds in the reactants
* Energy is released to form bonds in the products.
* Energy is released to form bonds in the products.
3
New cards
bond energies
The energy needed to break bonds and the energy released when bonds are formed can be calculated from bond energies.
4
New cards
overall energy change of the reaction.
The difference between the sum of the energy needed to break bonds in the reactants and the sum of the energy released when bonds in the products are formed is the overall energy change of the reactio
5
New cards
In an exothermic reaction,
the energy released from forming new bonds is greater than the energy needed to break existing bonds
6
New cards
In an endothermic reaction
the energy needed to break existing bonds is greater then the energy released from forming new bond
7
New cards
How do we calculate the overall energy change of a reaction
It is the difference between the sum of the energy needed to break bonds in the reactants and the sum of the energy released when bonds in the products are formed
reactants- products= overall energy change
\-x exothermic - burning
\+x endothermic
reactants- products= overall energy change
\-x exothermic - burning
\+x endothermic
8
New cards
bond breaking
energy in
9
New cards
bond making
energy out