biochem exam 2
5.0(1)
5.0(1)
Card Sorting
1/323
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
324 Terms
1
New cards
What are primary metabolites?
they are needed for normal operation of metabolic pathways and main cellular functions
2
New cards
What are secondary metabolites?
- organic compounds NOT needed for cell growth, development, or reproduction
3
New cards
What are the functions of secondary metabolites?
* ward off pathogens and predators
* protect against osmotic damage
* treat various illnesses
* protect against osmotic damage
* treat various illnesses
4
New cards
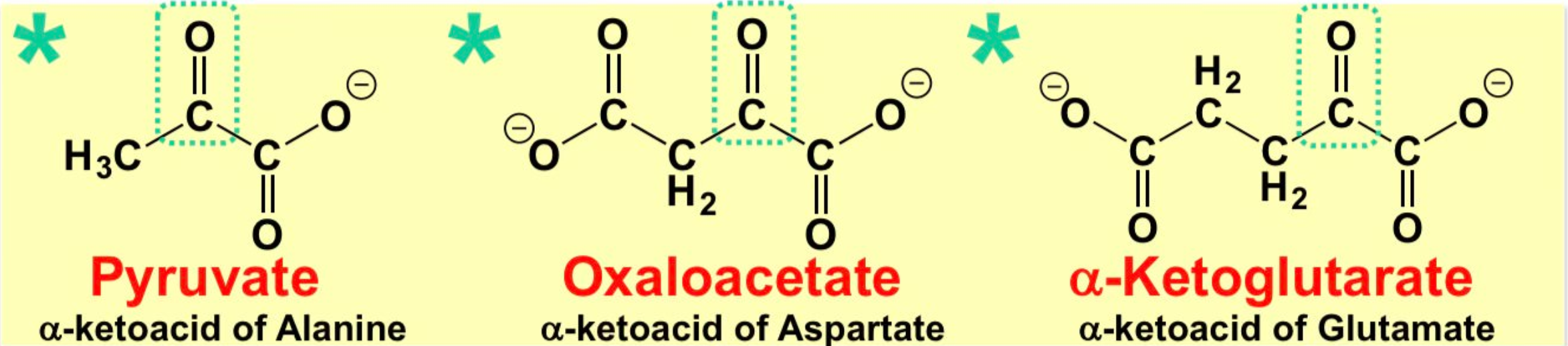
What are some examples of keto-acids?
pyruvate, oxaloacetate, a-ketoglutarate
5
New cards
Where is pyruvate found?
cytosol
6
New cards
Where is Oxaloacetate found?
* cytosol and mitochondria
* used in gluconeogenesis and citric acid cycle
* used in gluconeogenesis and citric acid cycle
7
New cards
Where is a-ketoglutarate found?
* mitochondria
* used in citric acid cycle
* used in citric acid cycle
8
New cards
What is the structure of pyruvate, oxaloacetate, and a-ketoglutarate?
\
9
New cards
What are the sources of amino acids?
* digestion of protein in food
* synthesis of amino acids
* prides aa's needed for protein synthesis
* adjust aa pools in different tissues
* adjusts energy metabolism by controlling levels of central pathway metabolites
* allow cells to adapt to metabolic stress
* needed to synthesize neurotransmitters and nucleotides
* synthesis of amino acids
* prides aa's needed for protein synthesis
* adjust aa pools in different tissues
* adjusts energy metabolism by controlling levels of central pathway metabolites
* allow cells to adapt to metabolic stress
* needed to synthesize neurotransmitters and nucleotides
10
New cards
What is the use of Amino Acids?
intracellular proteolysis:
* removes misfolded proteins
* removes old and damaged proteins
* regulates metabolism
* controls cell-cycle transitions
* removes misfolded proteins
* removes old and damaged proteins
* regulates metabolism
* controls cell-cycle transitions
11
New cards
"What are the 9 ""nutritionally essential (not made in body) amino acids?"
Phe, Val, Trp, Thr, Ile, Met, His, Leu, Lys
PVT TIM HiLL
PVT TIM HiLL
12
New cards
What are the conditionally essential amino acids?
* Arg: needed for growth childhood and pregancy
* Tyr: becomes essential when Phe is inadequate
* Cys: becomes essential when Met is inadequate
* Tyr: becomes essential when Phe is inadequate
* Cys: becomes essential when Met is inadequate
13
New cards
What is most Met presented as?
S-Adenosyl-Met
14
New cards
T or F: Omnivores are disadvantaged when is comes to getting a varied balance of amino acids in their diet.
F: they are advanataged
15
New cards
What is found in the 3 areas of proteolysis (enzymatic cleavage of proteins)?
* Saliva: proteases principally from bacteria and white blood cells
* stomach: pepsin
* intestine: neutral proteases like chymotrypsin (cleaves on the carboxyl end of aromatic residue), trypsin (cleaves on carboxyl end of lysine and arginine residues), carboxypeptidases (cleaves C-terminal AA), Elastase (cleaves elastin)
* stomach: pepsin
* intestine: neutral proteases like chymotrypsin (cleaves on the carboxyl end of aromatic residue), trypsin (cleaves on carboxyl end of lysine and arginine residues), carboxypeptidases (cleaves C-terminal AA), Elastase (cleaves elastin)
16
New cards
What is the path for proteolysis?
proteins --> stomach --> proteins that can't refold --> intestine --> amino acids + Di- and Tri-peptides
17
New cards
T or F: dietary proteins are absorbed by healthy intestines
F: must be proteolyzed to amino acids, di and tri- peptides
18
New cards
What begins enzymatic proteolysis?
making inactive proteases (zymogen)
\- synthesized and stored in pancreas
\- secreted into small intestine
\- converted to active catalysts
\- synthesized and stored in pancreas
\- secreted into small intestine
\- converted to active catalysts
19
New cards
What happens in trypsinogen activation?
* Pancreas makes and stores trypsinogen in vesicles
* secretory vesicles contain trypsin inhibitor (revents unwanted proteolysis of host cells)
* enterokinase, an ectoprotease on intestinal mucosal wall, converts trypsinogen into trypsin
* trypsin activates chymotrypsinogen
* secretory vesicles contain trypsin inhibitor (revents unwanted proteolysis of host cells)
* enterokinase, an ectoprotease on intestinal mucosal wall, converts trypsinogen into trypsin
* trypsin activates chymotrypsinogen
20
New cards
What is Pro-carboxypeptidase?
* activated to carboxypeptidase
* removes amino acids one-by-one from C-termini of food proteins
* removes amino acids one-by-one from C-termini of food proteins
21
New cards
Where does zymogen synthesis, processing, and transport occur?
ribosomes attached to rough ER -> golgi complex
22
New cards
Where does zymgen vesicale targeting and release occur?
zymogen granule
23
New cards
T or F: Zymogen formation prevents autophage and apoptosis
T
24
New cards
Where is pepsinogen formed in and released by?
gastric chief cells within the stomach
25
New cards
What does it mean by pepsinogen activation is autocatalytic?
once a little active pepsin forms, the latter catalyzes cleavage of many pepsinogen molecules to form more catalytically active enzyme molecules
26
New cards
How does pepsin operate at low pH?
by using its aspartic acid carboxyl groups for catalysis (aspartic proteinase)
27
New cards
T or F: turnover rate depends on metabolic state.
T: greater protein degradation, when nitrogen intake is low, because cells need amino acids to make vital proteins (ex: antibodies and hormones like insulin)
28
New cards
What is the lysosomal/phagolysosomal pathway?
* lysosome is an acidic compartment, where proteins undergo isoelectric expansion (partial unfolding
* low pH makes them more susceptible to proteolysis
* low pH makes them more susceptible to proteolysis
29
New cards
What is true nitrogen balance? positive? negative?
* True: intake = excretion
* Positive: intake > excretion
* Negative: excretion > intake
* Positive: intake > excretion
* Negative: excretion > intake
30
New cards
What is positive nitrogen balance required for?
* growth in childhood
* growth in pregnancy
* healing of wounds
* convalescence
* growth in pregnancy
* healing of wounds
* convalescence
31
New cards
When does negative nitrogen balance occur?
* starvation
* malnutrition
* disease (burns, trauma, surgery)
* malnutrition
* disease (burns, trauma, surgery)
32
New cards
What is marasmus?
* malnutrition associated with extensive tissue and muscle wasting, with little/no edema
* protein-energy malfunction resulting from inadequate intake of proteins and calories
* severe deficiency of nearly all nutrients, especially protein, carbohydrates, and lipids"
* protein-energy malfunction resulting from inadequate intake of proteins and calories
* severe deficiency of nearly all nutrients, especially protein, carbohydrates, and lipids"
33
New cards
What is Kwashiorkor?
* sickness last baby gets when the new baby arrives
* acute childhood protein malnutrition
* unlike marasmus, kwashiorker has inadequate protein intake, BUT otherwise adequate caloric intake
* charactertistics: irritability (neurotransmitter deficit), enlarged liver (fatty infiltrates), edema (caused by hypoalbuminemia)"
* acute childhood protein malnutrition
* unlike marasmus, kwashiorker has inadequate protein intake, BUT otherwise adequate caloric intake
* charactertistics: irritability (neurotransmitter deficit), enlarged liver (fatty infiltrates), edema (caused by hypoalbuminemia)"
34
New cards
Transaminases use \________ coenzymes
Vitamin B6
35
New cards
transaminases catalyze 2 half reactions
\
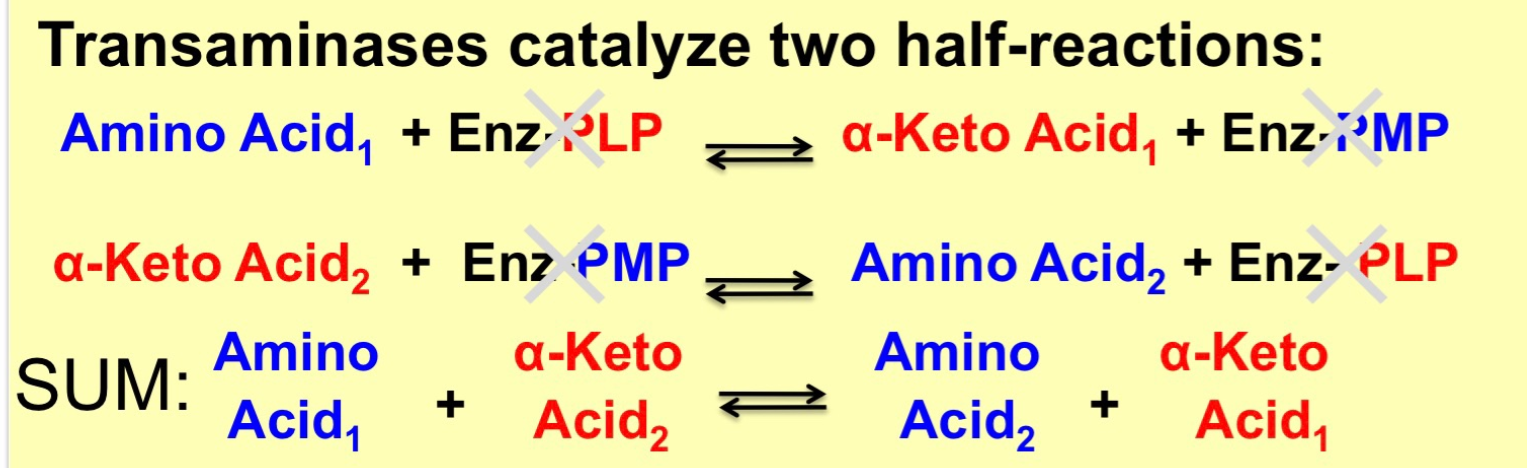
36
New cards
What is the mechanism of enzyme-bound reactions?
* 1st half-reaction: aldimine forms; converts to ketimine; hydrolyzes to ketoacid
* 2nd half-reaction: reverse of 1st half reaction, using R2-KA to make R2-AA
* 2nd half-reaction: reverse of 1st half reaction, using R2-KA to make R2-AA
37
New cards
aldimine vs ketamine
\
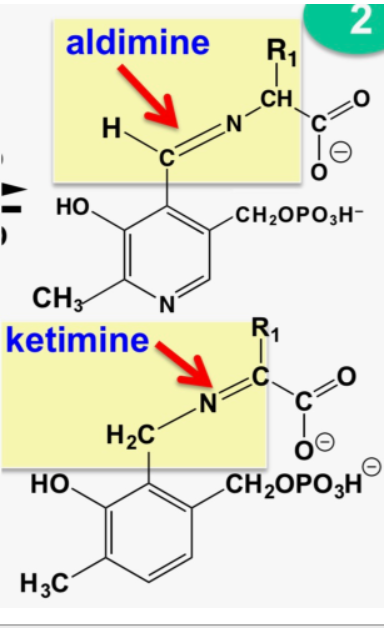
38
New cards
What is the Keq for transamination?
Keq= 1 (same bonding in substrates and products)
\-fully reversible
\-fully reversible
39
New cards
alanine: alpha- ketogluterate transaminase
\
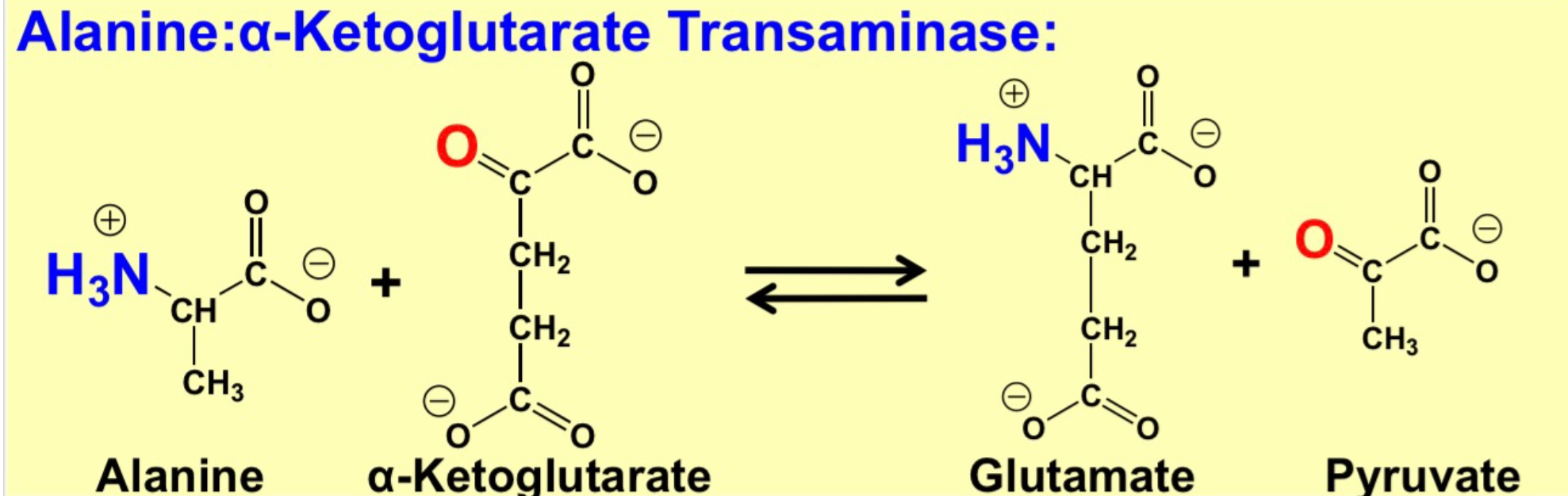
40
New cards
glutamate: oxaloacetate transaminase
\
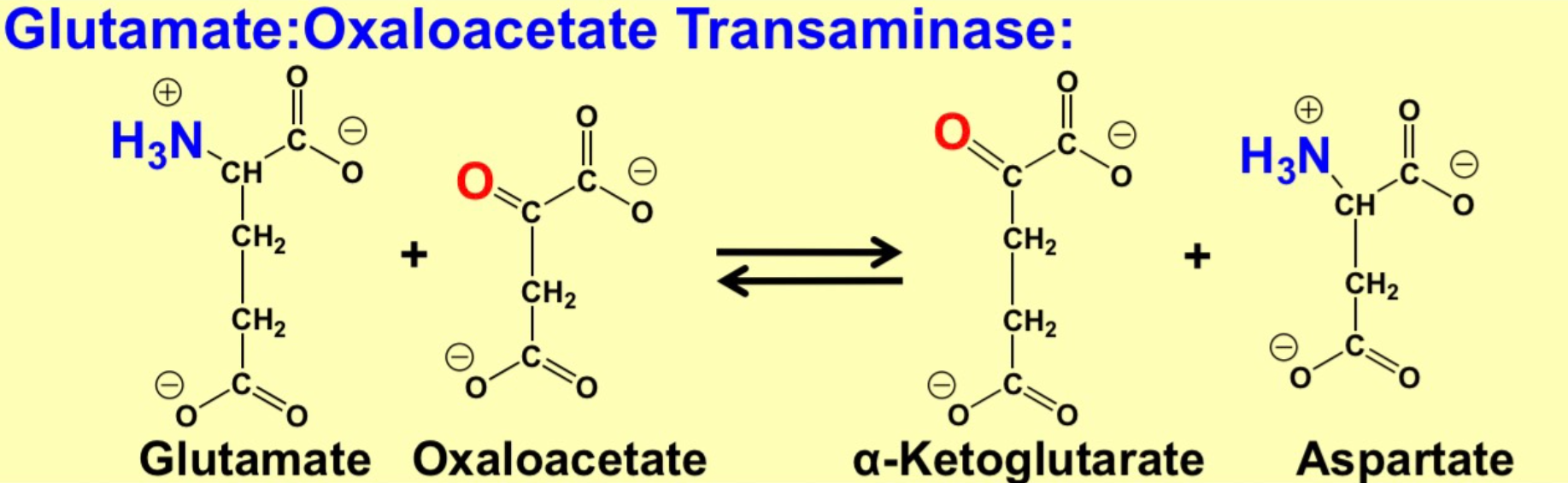
41
New cards
Which 4 amino acids CANNOT undergo transamination?
* proline and hydroxyproline: don't have the needed primary amine for transamination
* lysine: if transaminated, the keto acid would cyclize to form a toxic nonmetabolite
* threonine: if transaminized, the keto acid would dimerize into a toxic nonmetabolite
* lysine: if transaminated, the keto acid would cyclize to form a toxic nonmetabolite
* threonine: if transaminized, the keto acid would dimerize into a toxic nonmetabolite
42
New cards
T or F: transaminases cannot bind prolines and hydroxyprolines.
F: cannot bind lysine or threonine
43
New cards
What is the reaction equation foer glutamate dehydrogenase?
* major route for oxidative deamination
* glutamate + NAD^+ + H2O
* glutamate + NAD^+ + H2O
44
New cards
What is the structure for a-ketoglutarate?
\
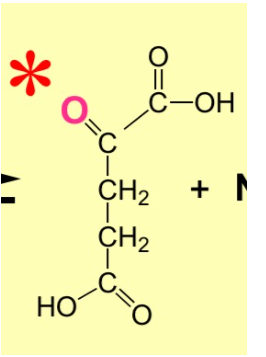
45
New cards
Where is glutamate dehydrogenase (GDH) located?
mitochondrial matrix
46
New cards
What are nature's batteries?
* NAD+ and NADH
* NADH oxidation yields enerfy needed to make 3 mol ATP from ADP and Pi through oxidative phosphorylation
* NADH oxidation yields enerfy needed to make 3 mol ATP from ADP and Pi through oxidative phosphorylation
47
New cards
What are the exceptions to the coupling of GDH with transaminases allowing for oxidative degradation of 14 other amino acids?
Pro, Hyp, Thr, Lys, His
48
New cards
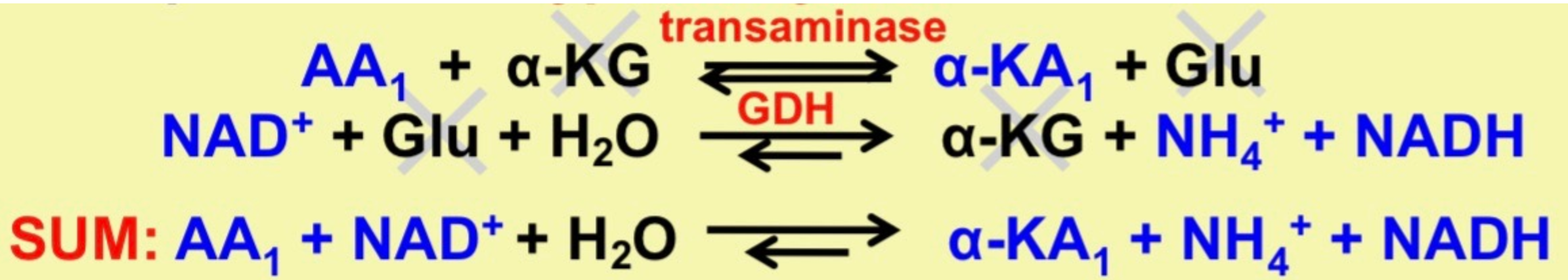
\
memorize
49
New cards
How does GDH use NAD+ in oxidative deamination reaction?
* NADH is re-oxidized to NAD+ in oxidative phosphorylation
* a-KA1 enters citric acid cycle
* excess NH4+ enters urea cycle.
* operating in the **opposite** direction,
* NADPH drives reductive amination to form glutamate.
* a-KA1 enters citric acid cycle
* excess NH4+ enters urea cycle.
* operating in the **opposite** direction,
* NADPH drives reductive amination to form glutamate.
50
New cards
GDH uses what?
NAD+and NADPH (NOT NADH and NADP+)
51
New cards
What happens when the mitochondria rapidly re-oxidizes NADH to NAD+?
* excess NAD+
* NAD+>>NADH
* NADH oxidation makes ATP in Mitochondria
* NAD+>>NADH
* NADH oxidation makes ATP in Mitochondria
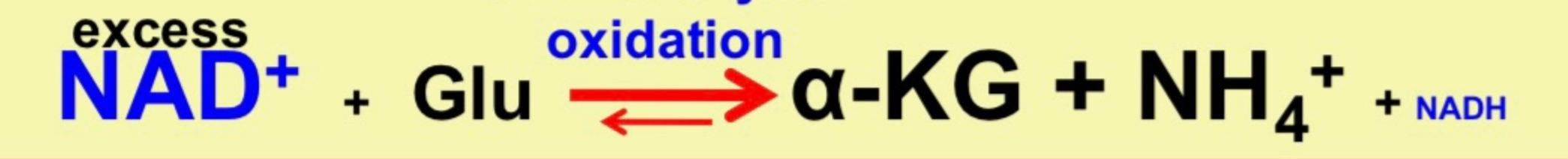
52
New cards
What happens when there is excess NADPH?
* NADPH>>NADP+
* NADPH is used to make fatty acids, sterols, etc.
* NADPH is used to make fatty acids, sterols, etc.
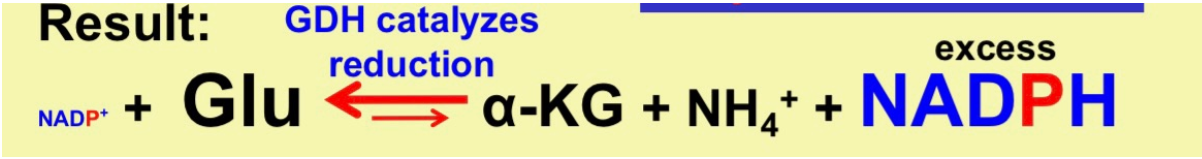
53
New cards
What inhibits GDH?
* high ATP, GTP, and NADH
* reduces AA degradation and favors protein synthesis
* reduces AA degradation and favors protein synthesis
54
New cards
What activates GDH?
* high ADP, GDP, and free amino acids
* a-KG stimulates citric acid cycle, fueling ATP synthesis
* a-KG stimulates citric acid cycle, fueling ATP synthesis
55
New cards
Glutaminase catalyzes \__________?
hydrolysis of glutamine
56
New cards
What is the glutaminase reaction equation?
glutamine + H2O ----> glutamate + NH3
57
New cards
Histidinase (or Histadine ammonia lyase) catalyzes \_________?
deamination of Histidine
histidine
histidine
58
New cards
Why do we need both L- and D- Amino acid oxidases?
* there are D- amino acids in damaged proteins
* D- amino acids are also abundant in old and dried food
* by forming ketoacids from D-AA, D- amino acid oxidase allows cells to harvest carbon skeletons as an energy source
* D- amino acids are also abundant in old and dried food
* by forming ketoacids from D-AA, D- amino acid oxidase allows cells to harvest carbon skeletons as an energy source
59
New cards
Asparaginase catalyzes \_____________?
hydrolysis of asparagine
asparagine -----> Aspartate + NH3
asparagine -----> Aspartate + NH3
60
New cards
What are the 4 enzymes that catalyze NH3 assmilation?
* Glutamate Dehydrogenase:
* NADPH + NH3 + a-KG ---> Glutamate + H2O + NADP+
* Glutamine Synthetase:
* ATP + NH3 + Glutamate ----> Glutamine + Pi + ADP + H+
* Carbamoyl-Phosphate Synthetase I:
* 2 ATP + NH3+ CO2 ----> H2N(C=O)-O-P+ Pi + 2ADP + H+
* NADPH + NH3 + a-KG ---> Glutamate + H2O + NADP+
* Glutamine Synthetase:
* ATP + NH3 + Glutamate ----> Glutamine + Pi + ADP + H+
* Carbamoyl-Phosphate Synthetase I:
* 2 ATP + NH3+ CO2 ----> H2N(C=O)-O-P+ Pi + 2ADP + H+
61
New cards
What is Glutamine Synthetase?
* Main way to trap NH3
* Gln is a major nitrogen source
* Gln is a major inter-organ nitrogen shuttle, avoiding direct transfer of NH3 from distant organs to liver
* Gln is a major nitrogen source
* Gln is a major inter-organ nitrogen shuttle, avoiding direct transfer of NH3 from distant organs to liver
62
New cards
What are the 2 SN2 reactions that occur in sequence for glutamine synthetase?
Glutamate ----ATP ADP---> gamma-Glutamyl-P (essential intermediate) ----> Glutamine
63
New cards
What drives ammonium ion deprotonation?
* ∆GATP-hydrolysis
* pK of ammonium ion deprotonation= 9.2
* ∆Gdeprotonation= 10 kJ/mol
* driven by ATP-dependent conformation change that moves guanidium group of a nearby arginine residue near enzyme-bound NH4+ to allow electrostatic repulsion to form NH3
* pK of ammonium ion deprotonation= 9.2
* ∆Gdeprotonation= 10 kJ/mol
* driven by ATP-dependent conformation change that moves guanidium group of a nearby arginine residue near enzyme-bound NH4+ to allow electrostatic repulsion to form NH3
64
New cards
What would happen if opposing enzymes were kept in the same compartment?
* futile cycles would pointlessly hydrolyze ATP
* Glutamine synthetase would convert glutamate to glutamine and glutaminase would convert glutamine to glutamate in a continuous cycle
* Glutamine synthetase would convert glutamate to glutamine and glutaminase would convert glutamine to glutamate in a continuous cycle
65
New cards
What is Carbamoyl-Phosphate Synthetase- I?
* main ammonia-assimilating reaction in mitochondria
* highly energy-dependent reaction
* 1st reaction resembles glutamine synthetase reaction and second reaction is a phosphorylation reaction
* highly energy-dependent reaction
* 1st reaction resembles glutamine synthetase reaction and second reaction is a phosphorylation reaction
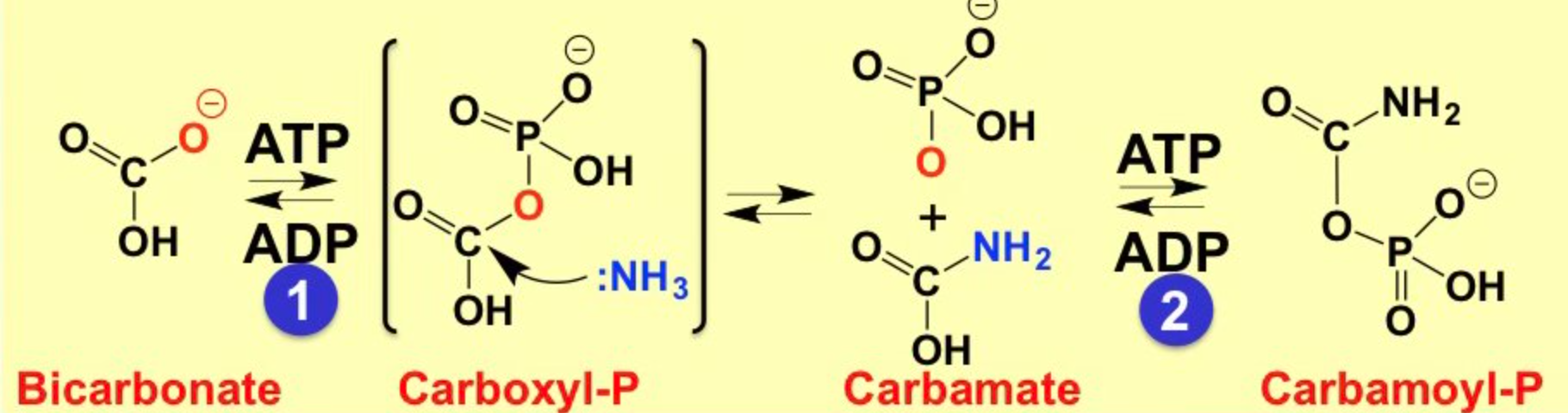
66
New cards
Why is glutamine vital for the efficient biosynthesis of nitrogen-containing metabolites?
* allows glutamine-dependent biosynthetic enzymes to generate ammonia *in situ*
* that ammonia is then used as a nucleophile without ever touching water
* that ammonia is then used as a nucleophile without ever touching water
67
New cards
What is CPS II?
* glutamine dependent
* uses a transfer tunnel to move unprotonated NH3 from glutamine-hydrolysis site to biosynthetic site
* covalently bound glutamate acts as a ""lid"" covering the glutamine site, preventing entry of H2O or H3O+
* used throughout metabolism
* uses a transfer tunnel to move unprotonated NH3 from glutamine-hydrolysis site to biosynthetic site
* covalently bound glutamate acts as a ""lid"" covering the glutamine site, preventing entry of H2O or H3O+
* used throughout metabolism
68
New cards
Which subunit in CPS-II hydrolyzes Gln to Glu + NH3?
glutamine-hydrolyzing subunit
69
New cards
T or F: The use of CPS-II allows are body to hydrolyze glutamine to deprotonate NH3 exactly where and when it is needed to avoid toxicity of ammonia elsewhere in out cells.
T
70
New cards
What are the characteristics of glutamine-dependent biosynthetic enzymes?
* exhibits a low Km (high affinity) for glutamine
* essential -SH group generates a gamma-glutamyl thioester that accupies active site, permitting NH3 transfer
* two active sites connected by ammonia transfer tunnel that prevents protonation of NH3 even at neutral pH
* essential -SH group generates a gamma-glutamyl thioester that accupies active site, permitting NH3 transfer
* two active sites connected by ammonia transfer tunnel that prevents protonation of NH3 even at neutral pH
71
New cards
What are the series of reactions that represent glutamine-dependent biosynthetic enzymes?
\
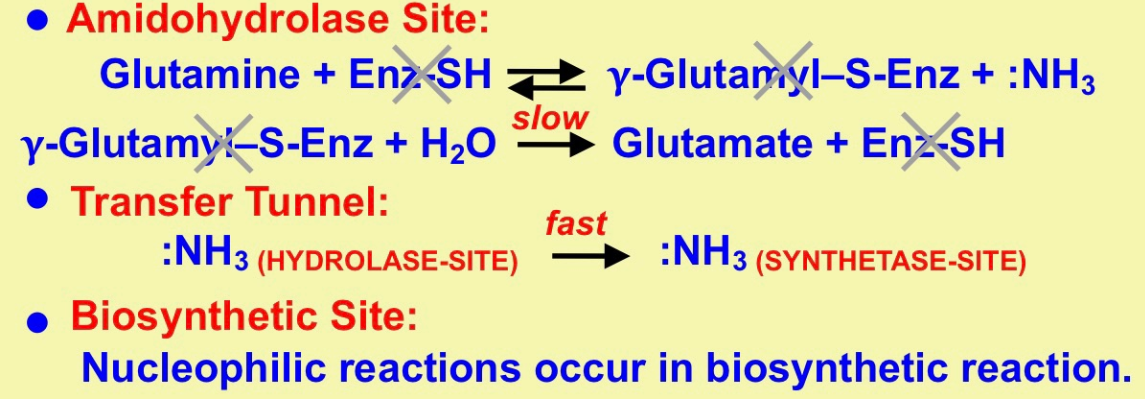
72
New cards
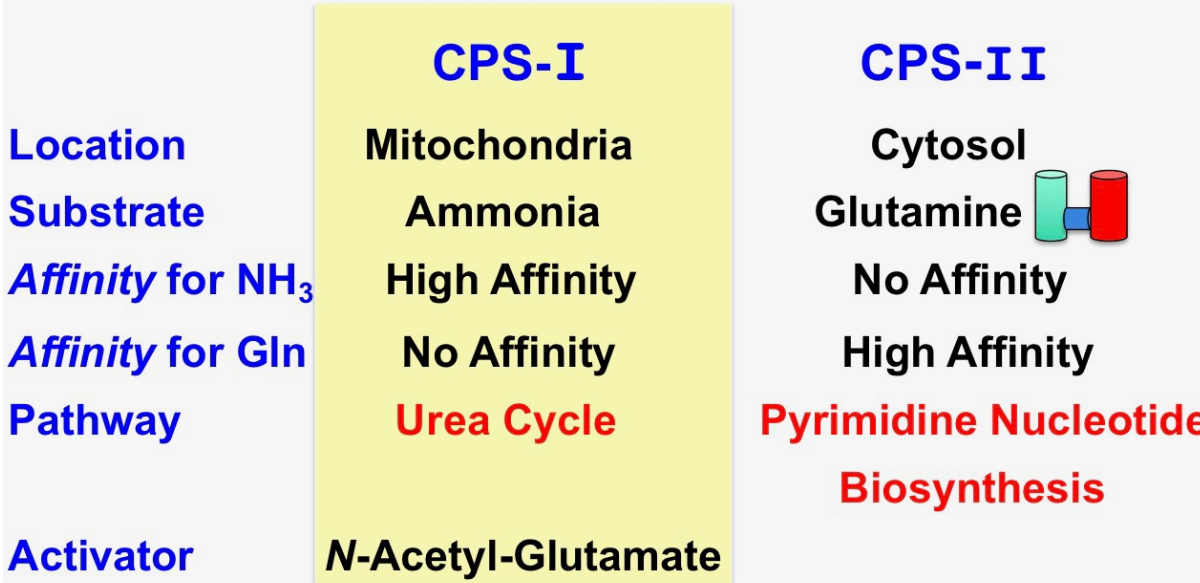
What is the differences between CPS I and CPS II?
\
73
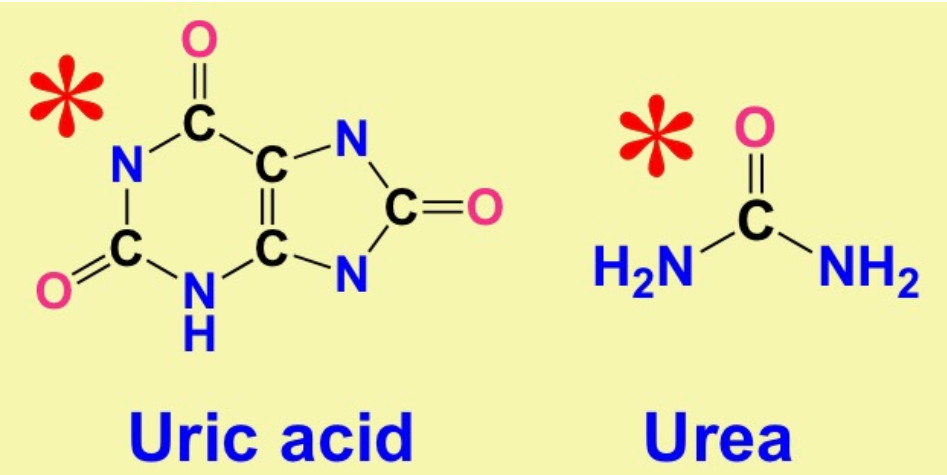
New cards
What is the structure of Uric Acid? What is the structure of ammonia?
\
74
New cards
What do fish excrete?
NH3 - Ammonotelic
75
New cards
What do birds excrete?
Uric Acid - Uricotelic
76
New cards
What do mammals excrete?
Urea - Ureotelic
77
New cards
What is gout?
Diseased caused by creating too much uric acid
78
New cards
What is the principal site of NH3 detoxification?
* liver
* high levels of ammonia in the blood is toxic so it is transported in organic forms like alanine, glutamate, glutamine, and urea
* high levels of ammonia in the blood is toxic so it is transported in organic forms like alanine, glutamate, glutamine, and urea
79
New cards
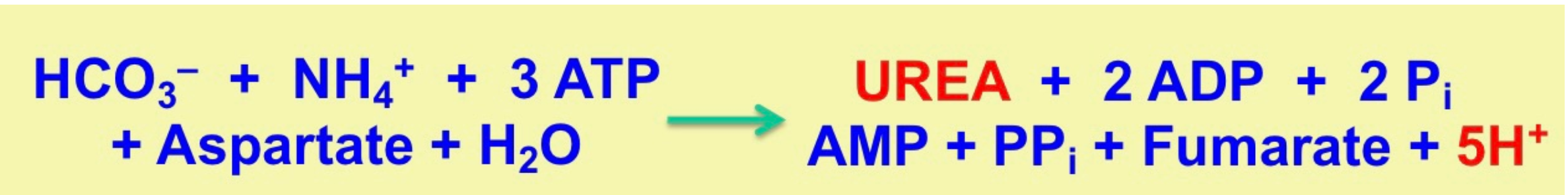
What is the overall stoichiometry for urea synthesis?
\
80
New cards
T or F: Urea cycle is an energy-independent pathway which liberates considerable acid to help control body pH.
F: Urea cycle is energy-dependent
81
New cards
What is the reaction equation for ornithine transcarbamoylase? Reaction mechanism for argininosuccinate synthase?
* Reaction: Aspartate + Gln + ATP
82
New cards
What is the structure for ornithine, citrulline, and arginino-succinate?
\
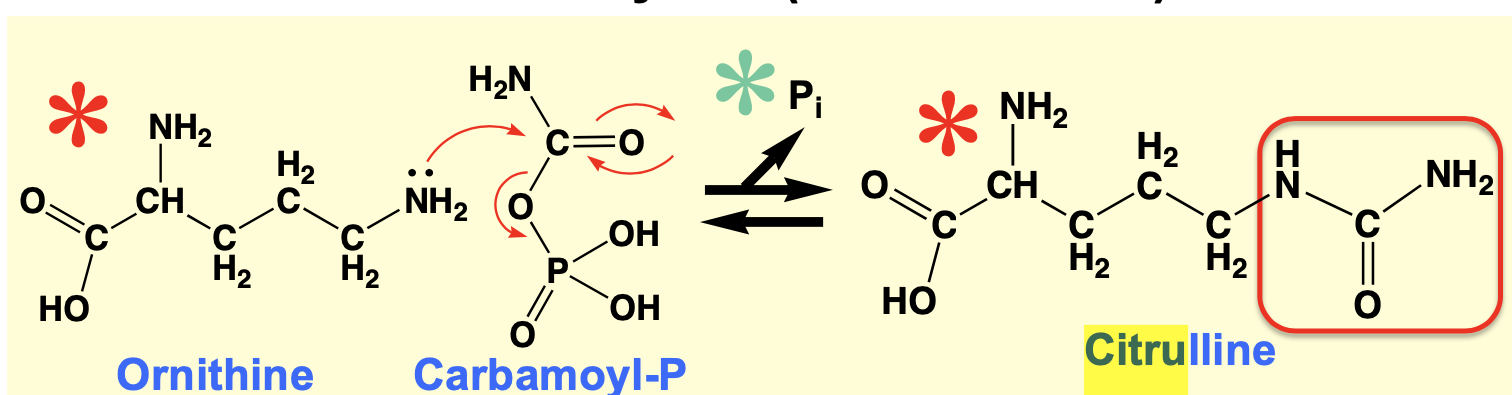
83
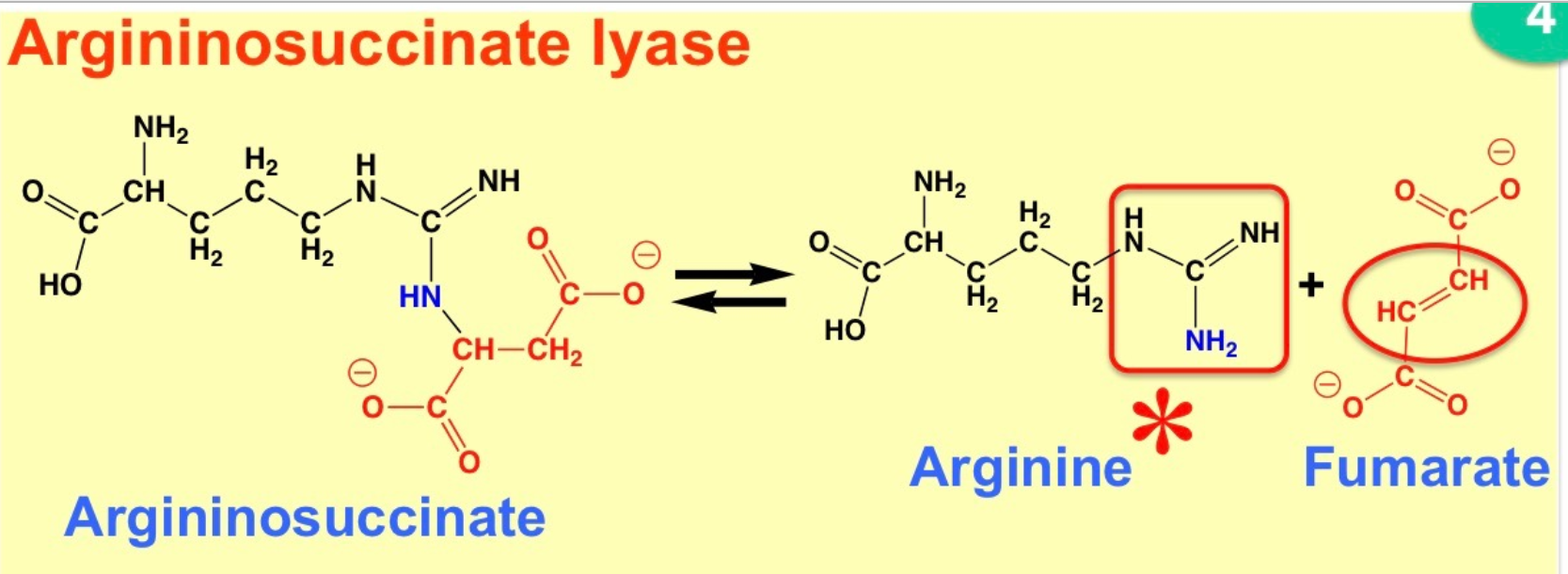
New cards
What is the reaction equation for argininosuccinate lyase?
\
84
New cards
Reaction equation for arginase?
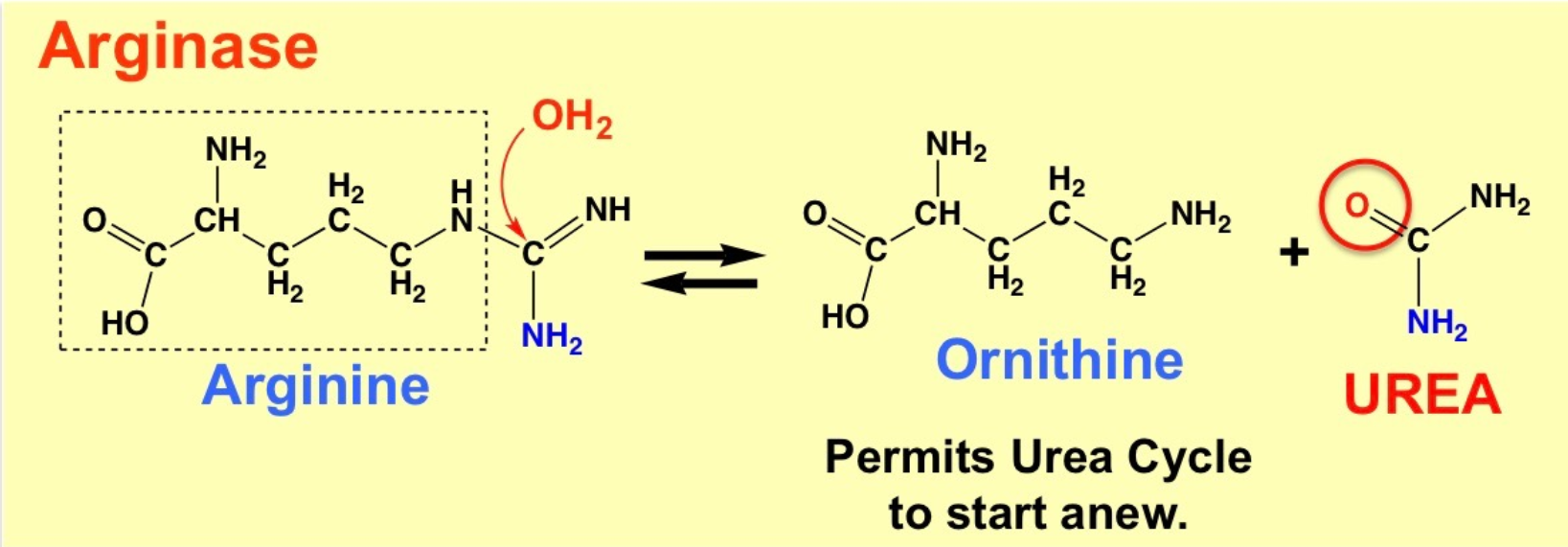
85
New cards
What is the Urea Cycle?
\
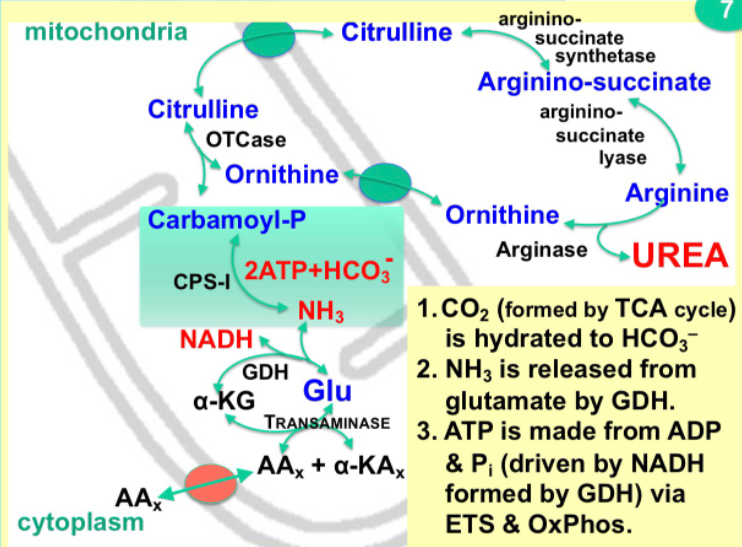
86
New cards
How does a high-protein diet induce urea cycle enzymes?
* certain amino acids stimulate glucagon release (casein or milk protein is rich in certain AAs)
* glucagon stimulates biosynthesis of urea cycle enzymes
* reduces ammonia load and increases pyruvate, OAA, and aKG for glucogenesis.
* glucagon stimulates biosynthesis of urea cycle enzymes
* reduces ammonia load and increases pyruvate, OAA, and aKG for glucogenesis.
87
New cards
How do arginine and glutamate regulate the urea cycle?
* CPS-I is the 1st commited step of the Urea Cycle
* CPS-I is allosterically activated by N-acetylglutamate.
* When amino acid catabolism increases, glutamate levels rise and so does N-acetyl-glutamate.
* CPS-I is allosterically activated by N-acetylglutamate.
* When amino acid catabolism increases, glutamate levels rise and so does N-acetyl-glutamate.
88
New cards
What is the reaction equation for NAGS (N-Acetyl-Glutamate Synthase)?
Glutamate + Acetyl CoA <\---> N-Acetyl-Glutamate + CoA
89
New cards
How are glutamine and arginine related to amino acid content and NAG?
* Glu and Arg are indicators of high amino acid content
* Glu is substrate for NAG synthase
* Arg activates NAGS allosterically
* NAG synthase deficiency results in hyperammonemia
* Glu is substrate for NAG synthase
* Arg activates NAGS allosterically
* NAG synthase deficiency results in hyperammonemia
90
New cards
How does the liver acinus avery ammonia toxicity?
* liver acinus is an ammonia trap
* endothelial lining within the liver is fenestrated (full of openings, exposing hepatocytes to blood)
* hepatocytes are in direct contact with blood components which permits rapid metabolite exchange between liver and blood
* endothelial lining within the liver is fenestrated (full of openings, exposing hepatocytes to blood)
* hepatocytes are in direct contact with blood components which permits rapid metabolite exchange between liver and blood
91
New cards
What does the liver acinus do?
- prevents ammonia re-entry into circulation through differential utilization of ammonia
92
New cards
Cells in the first segment of the acinar space contain \____________?
* glutaminase and urea cycle enzymes
* CPS-I has low affinity for NH3
* Urea cycle removes bulk of NH3
* Low affinity, High Capacity
* CPS-I has low affinity for NH3
* Urea cycle removes bulk of NH3
* Low affinity, High Capacity
93
New cards
After the blood goes through the first segment of the acinar space, blood passes through \____________, which are rich in \______________.
* perivenous scavenger cells; glutamine synthetase
* GS gas 40x higher affinity for NH3
* GS mops up NH3 that gets past the first segment
* Supplies glutamine to many organs
* High affinity, Low capacity
* GS gas 40x higher affinity for NH3
* GS mops up NH3 that gets past the first segment
* Supplies glutamine to many organs
* High affinity, Low capacity
94
New cards
How is Arginine synthesized?
\
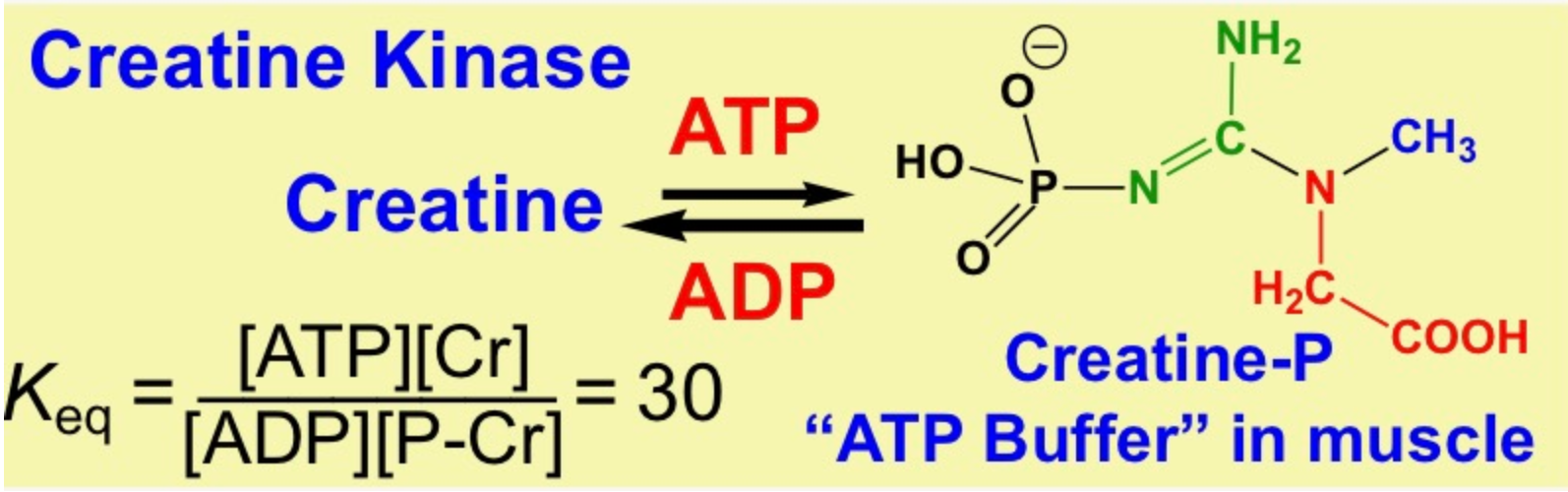
95
New cards
What is creatinine?
* break-down product of creatine-P in our muscles
* produced at a fairly constant uncatalyzed rate
* yield depends on muscle mass; higher in men
* clearance rate tells us how well kidney is working
* produced at a fairly constant uncatalyzed rate
* yield depends on muscle mass; higher in men
* clearance rate tells us how well kidney is working
96
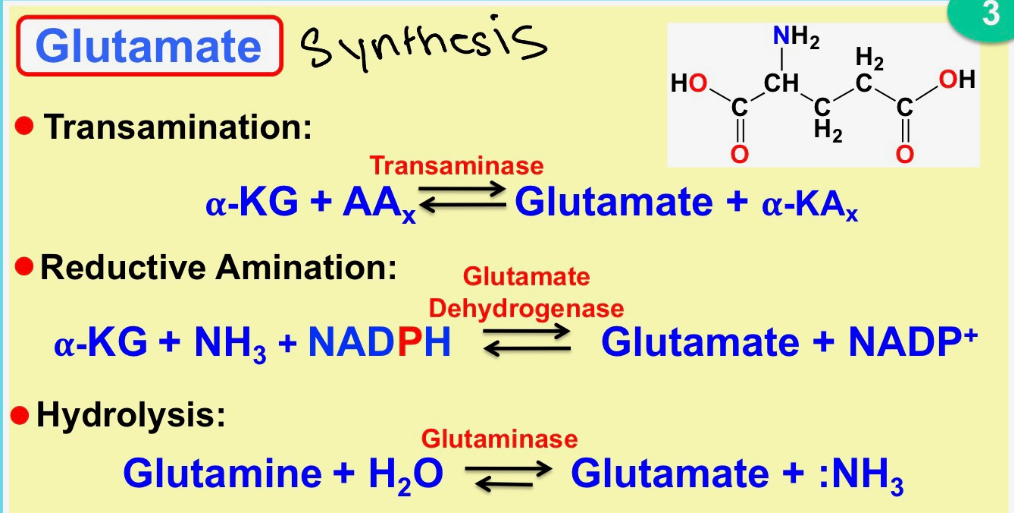
New cards
glutamate synthesis
transamination → reductive amination →hydrolysis
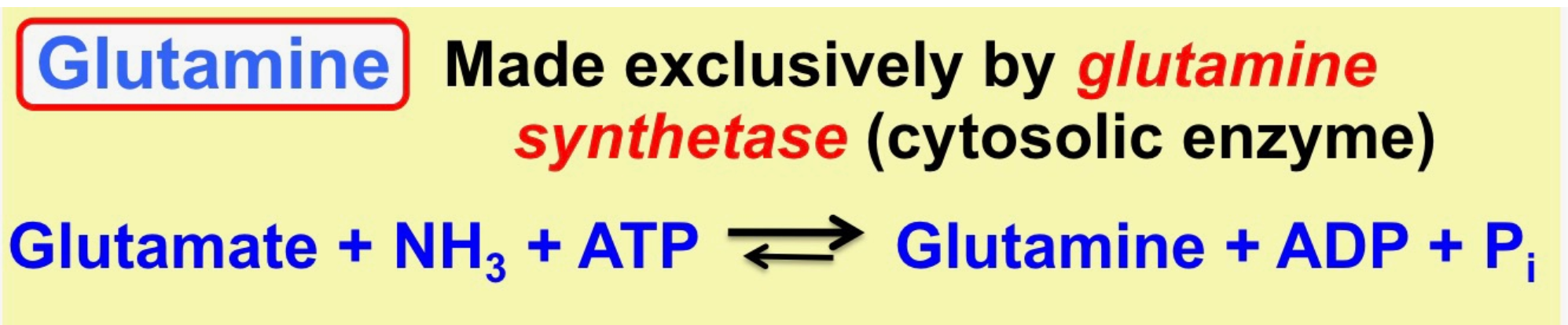
97
New cards
how is glutamine made?
\
98
New cards
What enzyme exclusively makes glutamine?
Glutamine synthetase (located in cytosol)
99
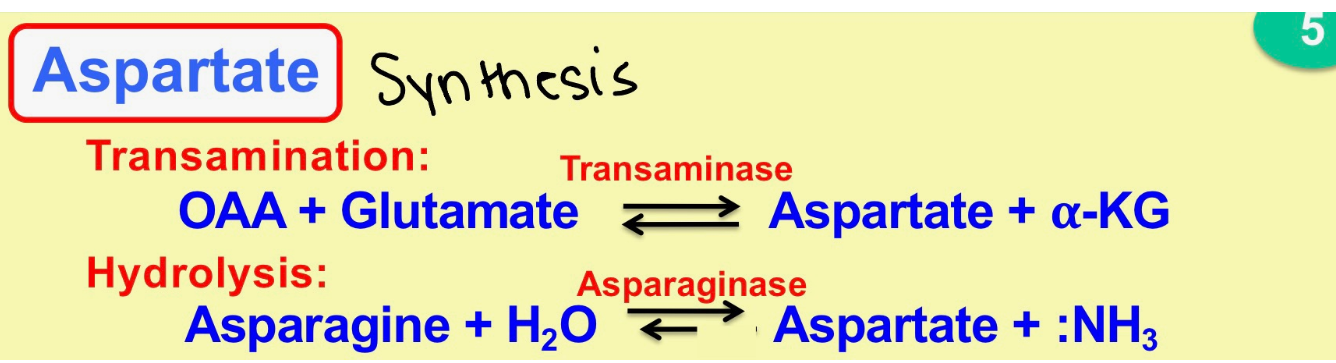
New cards
aspartate synthesis
transamintion → hydrolysis
100
New cards
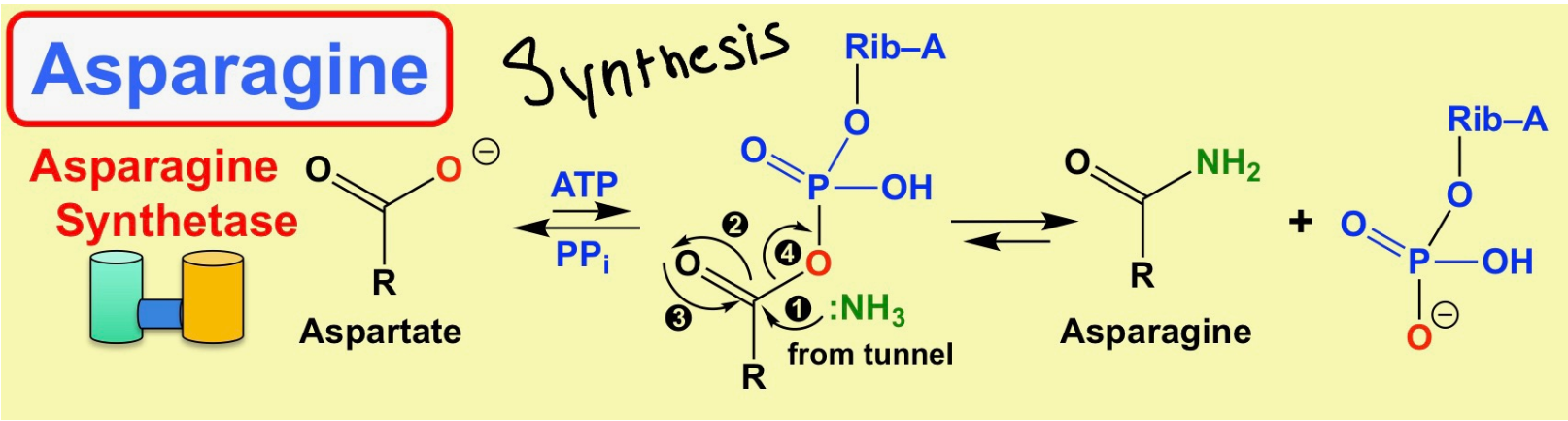
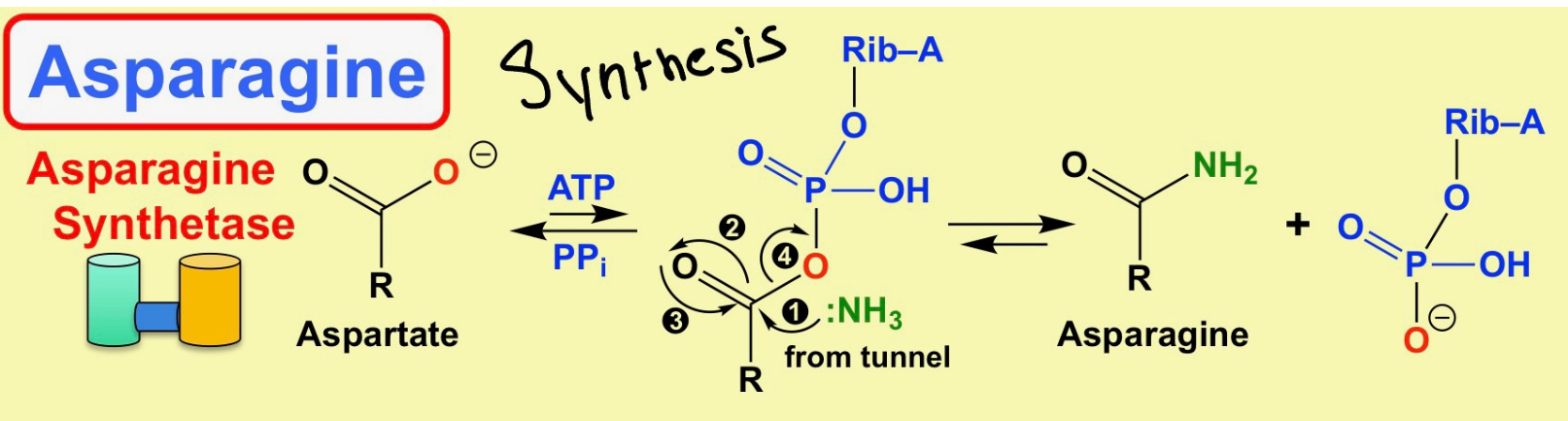
What is the reaction for Asparagine synthesis? Explain the reaction.
* Reaction: Aspartate + Gln + ATP