(pt 1) exam #5 - heme II (cls 546)
1/85
Earn XP
Description and Tags
secondary hemostasis testing + anti
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
end point detection methods in hemostasis (mechanical, photo-optical, nephelometric)
Mechanical
Detects fibrin strand
Photo-optical (turbidometric)
detects change in optical density of the plasma as the clot forms
Nephelometric
Uses FSC and SSC to detect clot formation
end point detection methods in hemostasis (chromogenic, immunologic, viscoelastic)
Chromogenic (amidolytic)
Measures activity of a specific factor (substrate based)
Spectrophotometry (405nm)
Immunologic
Based on Ag-Ab reaction and light scatter end point detection
Viscoelastic
Whole blood clotting – TEG
Measures whole clotting process – kinetics, strength, fibrinolysis
3 different levels of coagulation automation
manual
semiautomated
automated
what type of instrument is the fibrometer? end-point detection?
semi-automated ; mechanical detection
what tests do we use to evaluate disorders of secondary hemostasis?
PT, PTT, TT, FIB
1:1 mixing study, specific coag factors, reptilase time (RT), prekallikrein, factor XIII, VWF assays, russell viper venom time (dRVVT), inhibitor assays
screening tests of secondary hemostasis
Prothrombin time (PT)
Activated partial thromboplastin time (APTT)
Thrombin time (TT)
Quantitative fibrinogen
what type of specimen is used for coagulation testing?
platelet poor plasma ; primarily sodium citrate
what is platelet poor plasma? why platelet poor plasma?
plasma w <10 x 10^3 /uL platelets
Platelets have: PF4--neutralizes heparin
Phospholipids--affect factor assay
Proteases--affect vWF
specimen issues that can occur and their effect on coagulation testing
short draw—affects dilution of blood in sodium citrate tube
clot in specimen—unacceptable & cannot run the test
hemolysis—in vitro activation of plts and coagulation, results unreliable
lipemia/icterus—interfere w optical detection of clots
prolonged tourniquet application—elevates conc of VWF & VII; shortens time on clot-based tests
storage @ 1-6 C—cause ppt of large VWF & destroys platelet integrity
storage @ >25 C—factor VIII deterioration
what are we detecting in the PT and PTT tests?
formation of a clot (fibrin strands)
what pathway is evaluated with the PT? PTT?
PT = extrinsic + common
PTT = intrinsic + common
what is the PT most commonly used for? what else can prolong a PT?
Monitoring oral anticoagulant coumadin/warfarin
Vitamin K deficiency
what is added in the PT testing system?
Thromboplastin (TF/Ca2+)
Comes from rabbit brains
what are the causes of prolonged PT's?
Oral anticoagulant therapy
Deficiencies in factor VII, X, V, II
Fibrinogen inhibitors ; vit K deficiency
wow low does a factor activity have to be before it is detected as deficient in a PT or PTT?
25-40% of normal
what is added in the PTT testing system?
activated partial thromboplastin + Ca
activated partial thromboplastin time (APTT)
Partial thromboplastin
Provides phospholipid surfaces
Stimulates activated platelet surfaces
Activator (kaolin, celite, micronized silica, ellagic acid)
Negatively charged surface for activation of FXII and PK
what are the causes of prolonged PTTs?
Heparin therapy ; deficiencies in factor XII, XI, IX, VIII, X, V, II
fibrinogen, PK, HMWK inhibitors
what does the thrombin time (TT) test?
conversion of fibrinogen → fibrin
what is added in the TT testing system? reference range?
thrombin ; 15-19s (TUKHS 12.6-17.0)
causes of prolonged thrombin times?
Afibrinogenemia (or hypo) ; dysfibrinogenemia
Heparin therapy ; thrombin inhibitors
DIC (FDPs
what do the thrombin time and reptilase time tests have in common?
thrombin time and reptilase times bypass both intrinsic/extrinsic pathways
test for the polymerization of fibrin or conversion of fibrinogen to fibrin
prolonged thrombin time due to what? (detailed)
Interference in conversion of fibrinogen to fibrin
Hypofibrinogenemia or dysfibrinogenemia
Presence of heparin, direct thrombin inhibitors (DTIs) or FDPs (interfere w fibrin formation)
Rare cases
Autoantibodies against thrombin, paraproteins (myelomas)
causes of extremelely long thrombin time (TT)?
Usually indicates heparin effect
Neutralized w hepzyme and repeat testing
what is a reptilase time?
used w TT to detect heparin contamination
differentiates dysfibrinogenemia from FDP & paraproteins
reptilase time (general)
Reptilase is a serine protease
Found in venom of Bothrops atrox snake
Cleaves fibrinopeptide A from fibrinogen
Thrombin cleaves both fibrinopeptide A and B
Addition of reptilase to PPP
Initiates clot formation
Detected by optical or electromechanical methods
Manual, semi-automated, automated methods
General reference interval
18-22 seconds
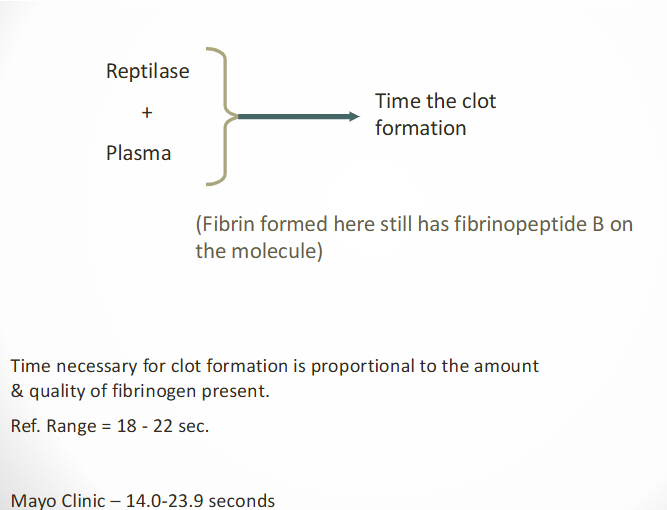
increased reptilase times are due to what?
dysfibrinogenemia, hypofibrinogenemia, afibrinogenemia
usually >25 seconds or longer
difference between thrombin time and fibrinogen assay?
Both use thrombin reagent
TT is functional ; fibrinogen is quantitative
what does the fibrinogen assay test?
amount of fibrinogen present
quantitative fibrinogen assay (general)
Reference method: Clauss assay
Clot based functional measurement
Adds thrombin to various dilutions of known concentrations of fibrinogen
Clotting times are plotted on a log/log graph
X-axis--known concentrations
y-axis--clotting times
Fibrinogen concentration inversely proportional to the clotting time
Determined from reference curve
RR = 200-400 mg/dL ; critical <100 mg/dL
(quantitative fibrinogen assay) what dilution is used in the fibrinogen assay for the patient and controls? why?
1:10 ; prevents interference from FDPs
(quantitative fibrinogen assay) when you get a low fibrinogen result with a 1:10 dilution, what do you do?
make a smaller dilution --1:2 or 1:5
divide by dilution factor!
what are the causes of decreased fibrinogen?
DIC ; primary and secondary fibrinolysis
Liver disease ; inherited diseases
what are the causes of increased fibrinogen?
Inflammatory disorders
Pregnancy ; oral contraceptives
Cardiovascular disease
quantitative fibrinogen ANTIGEN assay
Fibrinogen antigen tests
Immunoturbidimetric method
Immunoprecipitin analysis
Sample mixed with ABY to FIB
Detects dysfibrinogenemia vs afib or hypofib
results interpretation of quantitative fibrinogen ANTIGEN assay
Activity & antigen low = hypofibrinogenemia
Absent = afibrinogenemia
Activity low & antigen normal = dysfibrinogenemia
normal range for a fibrinogen antigen = 196-441 mg/dL
tests to evaluate specific factor deficiency (general)
Further testing performed if
PT and/or APTT is prolonged
Mixing studies: Tell us if it is an inhibitor or a deficiency by adding in factors (pooled normal plasma)
Specific coagulation factors
Reptilase time
Prekallikrein screening test
Factor XIII screening test
VWF tests
what is a mixing study or 1:1? what does it do?
Add pt sample to pooled normal plasma (PNP) OR specific factor deficient plasma (specific coag factor assays)
Function: differentiates factor deficiencies from inhibitors
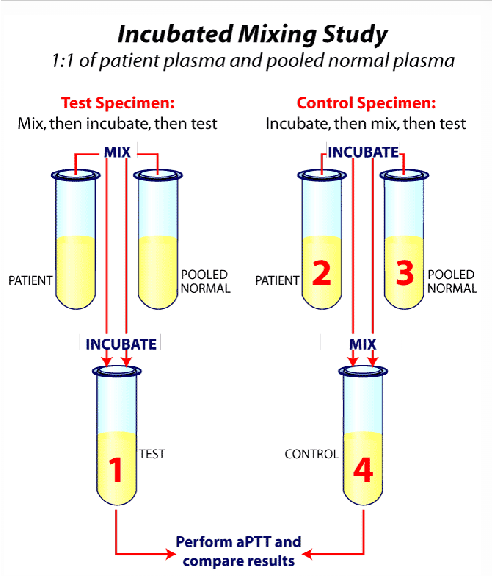
(1:1 mixing studies) you add PNP to your sample; it corrects to a normal PTT. what is the problem with the patient sample? why?
Factor deficiency
adding a 1:1 mix means that you are increasing the pt's deficiency to over the 50% mark where it can be detected
(1:1 mixing studies) you add PNP to your sample & the PTT is still abnormal. what is the problem with the patient sample? why?
Inhibitor present
PNP has coag factors already, so there is something in the pt sample that is preventing clot formation
other names for mixing studies
Circulating anticoagulant screen
Screening test for circulation inhibitor
Factor VII inhibitor screen
Factor X inhibitor screen
1:1 Mix
results of mixing studies
Patient's plasma corrected after incubation with normal plasma
Factor deficiency
Normal plasma replenishes the deficient factor in patient's plasma
Next step: perform factor assays
Patient's plasma NOT corrected after incubation with normal plasma
Presence of circulating or specific factor inhibitor
Next step: perform test to verify type of inhibitor
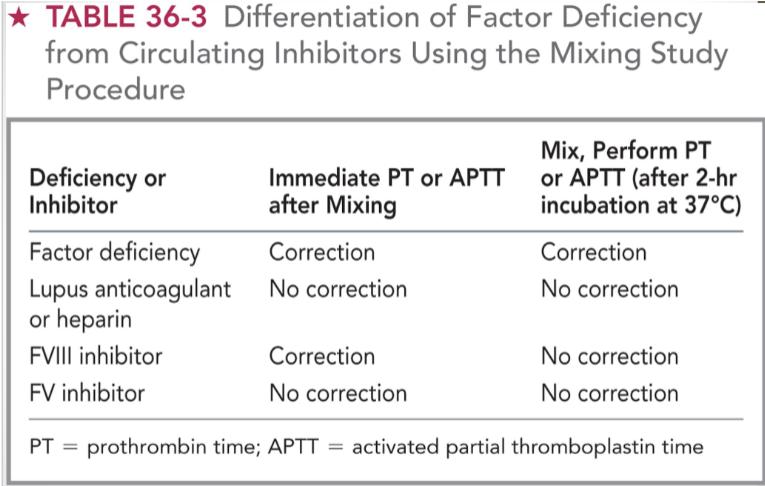
mixing study results interpretation (chart)
factor deficiency = immediate correction + correction after 37 C incubation
lupus anticoagulant/heparin = no immediate correction + no correction after 37 C
FVIII inhibitor = immediate correction + no correction after 37 C
FV inhibitor = no immediate correction + no correction after 37 C
acquired blood clotting disorders occur in what conditions?
Vitamin K deficiency
Liver diseases
Liver transplantation
Disseminated intravascular coagulation
Renal disease
Primary pathological fibrinolysis
During the course of anticoagulant therapy
specifc coagulation factor assays (function, principle, etc)
Performed to:
Confirm specific factor deficiency
Determine actual activity of factor
Principle
Ability of a patient's plasma to correct a prolonged PT or APTT of a known factor-deficiency plasma (substrate)
One-stage assays based on the PT
Extrinsic (FVII) and common pathway (FII, FV, FX)
One-stage assays based on the APTT
Intrinsic pathway (FVIII, FIX, FXI, FXII)
(coag factor tests) possible tests for factor X
1 stage PT-based assay (common pathway)
1 stage PTT-based Assay (Common pathway)
Chromogenic Factor X assay
Immunological Factor X assay
Russell Viper Venom assay
Venom activates Factor X
procedure for factor assays
Standard curve constructed from:
Clotting times of factor deficient substrate plasma containing varying dilutions of a reference pooled plasma
Patient's clotting time
1:10 and 1:20 and/or 1:40 dilutions of patient plasma with specific factor-deficient plasma
Perform PT or APTT on mixture (depending on the factor)
Times are converted to % activity from standard curve
Results should be linear
If not--suggests inhibitors
Normal factor activity reference interval
~50-150%
(factor assays) prekallikrein screening test
Patients with PK (Fletcher factor) deficiency
Have a prolonged APTT
Correction of prolonged APTT to normal after:
10 minute incubation period before adding calcium chloride
Longer incubation--increased contact activation of FXII
Confirmed with specific factor assay using PK-deficiency substrate
factor XIII screening test
aka 5m urea solubility test
Factor XIII = fibrin stabilizing factor
Necessary for stable fibrin clot formation
Occurs by forming covalent bonds between fibrin monomers
Screening test principle
Fibrin clot has increased solubility because of the lack of cross-linking of fibrin polymer in absence of FXIII
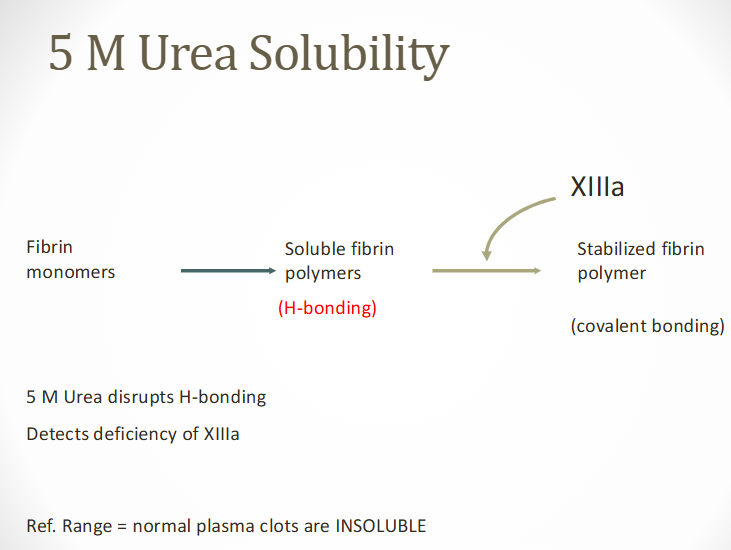
(factor XIII screening) 5M urea procedure
Patient's PPP is clotted with 0.025M CaCl2
Allowed to clot for 1 hr at 37 C
Clot is placed in 5M urea of 1% monochloroacetic acid in 37 C water bath
If Factor XIII deficient:
Patient's clot dissolves within 24 hour period
Indicates activity <1-2%
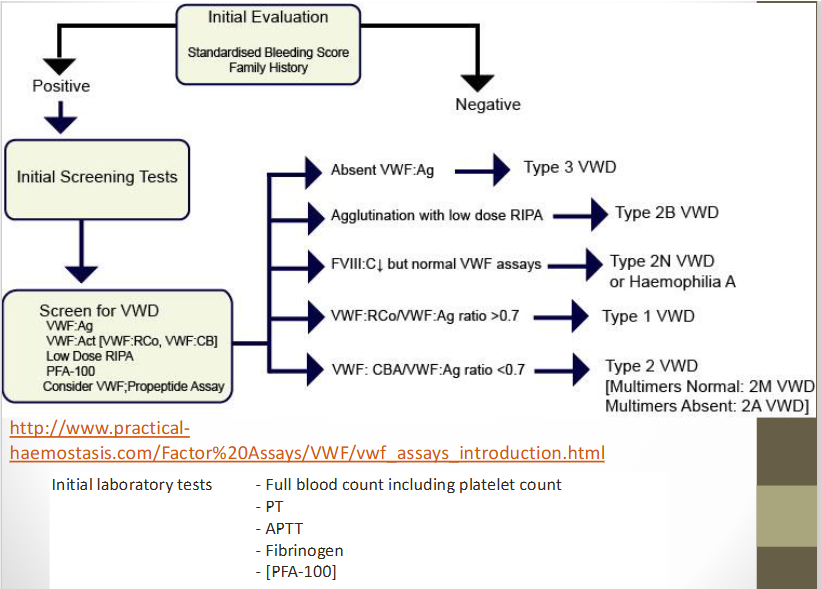
3 major categories of vWF deficiency? what does each category represent?
Type 1 VWD: partial quantitative deficiency of VWD ; most common
Type 2 VWD: qualitative deficiency of VWD
Type 3 VWD: complete absence of VWD
variables affecting VWF
Endogenous release of adrenaline
Can result in transient FVIII and VWF increase (2-3x)
Processing of citrated sample
Must be PPP with platelet count <10,000/uL
Patient's blood type
Type O has lower VWF activity
Variety of clinical disorders
Increased levels with inflammation, pregnancy, birth control pills
von willebrand’s disease (VWD)
Requires a panel of quantitative and functional assays
VWF antigen (VWF:Ag) immunoassay
Clot based factor VIII assay
Functional VWF ristocetin cofactor (VWF:RCo) REAGENT PLT
Dilute ristocetin-induced platelet aggregation assay (RIPA)
Patient PLT
Aka--ristocetin response curve
Used for VWD subtype 2B
Immunoassays
VWF activity enzyme immunoassay
VWF collagen binding assay
definitive diagnosis of VWD requires what?
Quantitative VWF by both functional and antigenic methods
VWF:A—Activity (aka Ristocetin cofactor assay)
Functional assay
Uses a monoclonal ABY that targets GPIb binding
VWF:Ag—Antigen
Immunological assay
Quantitative measure of vWF in plasma
Variables that affect accurate determination difference over time in VWF results in patients with VWD
Sometimes VWF:Ag and/or VWF:A levels will be normal
Results of screening tests can be normal in type I VWD
Single evaluation cannot rule out VWD
Patients with history of bleeding--repeat testing is recommended
treatment for classic VWD
Desmopressin (Desamino-D Arginine vasopression-DDAVP)
Causes two-fold to five-fold ↑ in patients VWF plasma level in type I patients
Test dose of DDAVP is given to patient prior to surgery
Citrated plasma samples are drawn
Pre-infusion
30 minutes post-infusion
4 hours post-infusion
Assayed for FVIII activity, VWF:Ag, VWF:A
tests for VWD activity (VWF:A) (2)
Ristocetin cofactor (RCoF) assay
In presence of ristocetin (reagent platelets)
VWF induces platelet agglutination
Measured on platelet aggregometer (ristocetin induced platelet aggregation – RIPA abnormal in BSS and VWD)
Quantitative test for VWF activity (VWF:A)
Uses ristocetin to induce VWF to bind to glycoprotein IB/IX receptor on formalin fixed platelets
Eliminates variability of patient's GPIB/IX
Measured on platelet aggregometer
Reference standard curve
tests for VWF antigen (VWF:Ag)
Laurell based assay
sandwich ELISA
LIA w latex particles
(VWF:Ag test) laurell based assay
EIA that electrophoreses the plasma sample through an agarose gel
Agarose gel contains rabbit anti-serum to FVIII
Rocket-shaped immunoprecipitate forms
Length is directly proportional amount of VWF:Ag
Time-consuming and difficult to measure decreased levels
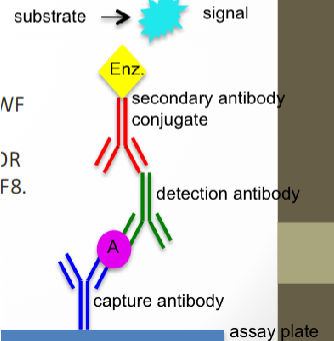
(VWF:Ag test) sandwich ELISA
Microtiter plate wells coated with specific antihuman VWF antibody
Binds to VWF:Ag in test sample
Second rabbit antihuman VWF:Ag antibody is enzyme labeled
Binds to initial antigen-antibody complex
VWF protein is sandwiched between two specific Abs
Substrate is added--colorimetric reaction
Color intensity directly proportional to concentration
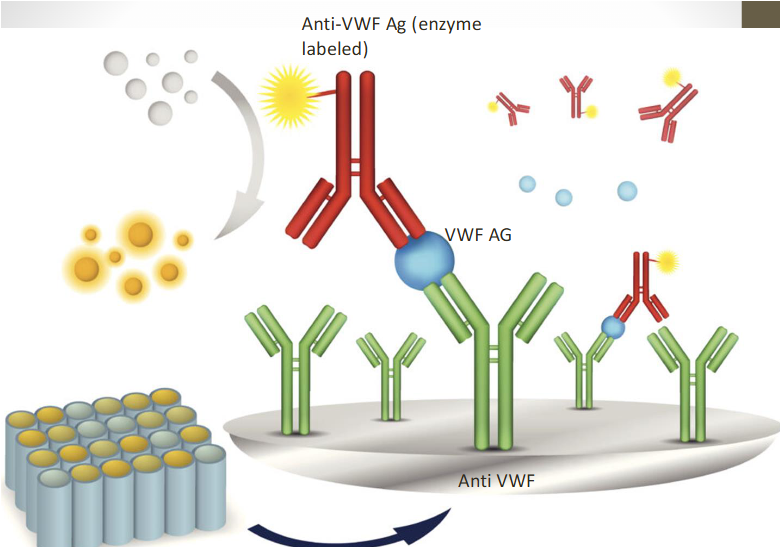
(VWF:Ag test) LIA w latex particles
New LIA assay
Uses Latex particle-enhanced Immunoturbidometric Assay (LIA) to quantify VWF:Ag
Measures turbidity produced by agglutination of latex reagent
Specific anti-VWF monoclonal antibody to GPIb binding site on VWF is absorbed to latex reagent
Reacts with VWF in patient plasma
Degree of agglutination is directly proportional to VWF:A
Reliable, easy to automate, and timely
Can also detect low levels of antigen
Reference interval VWF:A 60–150%
what is VWF:FVIIIB? how is it measured?
lab test for factor FVIII binding assay VWF:FVIIIB
Measures the binding of FVIII to VWF
96 well plates coated with anti-human VWF
incubated with plasma samples
Peroxidase-conjugated anti-vwf (sandwich) OR peroxidase conjugated anti-human F8
Std curve with dilutions of PNP
Color generation is detected
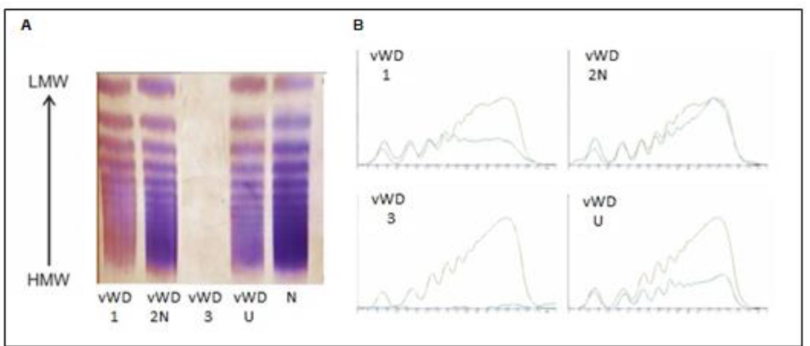
VWF multimer analysis
Used to determine correct disease subtype
Gel electrophoresis
Low concentration of agarose (0.65%)
Staining w Ab to VWF
Type is determined by electrophoretic pattern
Also useful for diagnosing TTP
Presence of unusually large VWF (UL-VWF) multimers indicates decreased ADAMTS-13 activity
Performed in specialized reference labs or coagulation centers
collagen binding assays for VWD (VWF:CB)
Specialized assays
Differentiate VWD type 2A and 2B from 2M
ELISA assay
Perform both collagen binding assays and VWF:A assays
Increases ability to differentiate VWD type 2 variants
Only 2A and 2B have abnormal VWF:CB
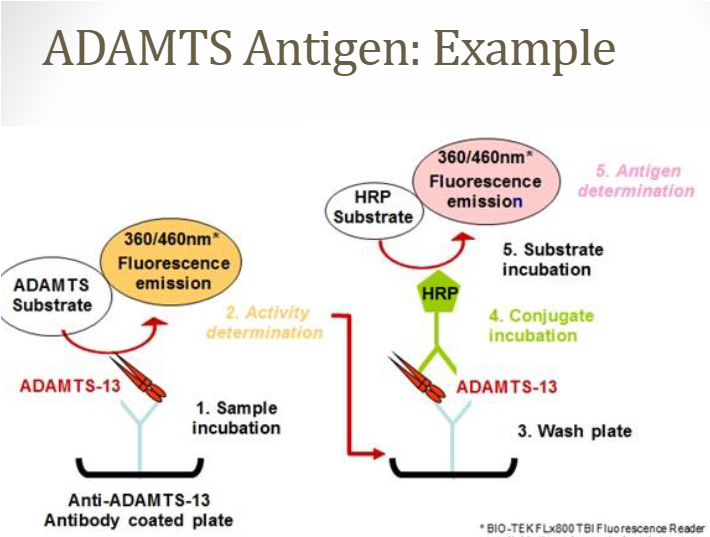
(VWF) ADAMTS-13
VWF cleaving protease
Methodologies
ELISA, fluorescence resonance energy transfer (FRET), and other direct or indirect assays available
Measure ADAMTS-13 activity, antigen, or autoantibody levels
Use citrated PPP (platelet poor plasma – blue top)
what are the VWF tests used to differentiate the different types of VWD? (see chart)
circulating anticoagulants (inhibitors)
Acquired pathologic plasma proteins that inhibit normal coagulation
Primarily immunoglobulins
Produced in response to variety of stimuli
Blood or blood products
Release of tumor substances into circulation
Autoimmune disorders
types/action of inhibitors in hemostasis
Inhibitor's action may be:
Confined to a specific factor (ex VIII inhibitor)
Nonspecific (ex: lupus anticoagulant, which is anti-phospholipid)
Global, affecting several factors simultaneously (ex: heparin
immediate acting vs time dependent hemostasis inhibitors
Immediate acting
Heparin, lupus anticoagulant, factor IX inhibitor
Time dependent
Factor VIII inhibitor
conditions associated with inhibitors
Hemophilia A (VIII) and hemophilia B (IX)
DIC ; pregnancy
SLE, Waldenstrom's macroglobulinemia, plasma cell dyscrasias
Elderly patients
ID of common circulating inhibitors
Most common circulating inhibitors (2)
Lupus-like anticoagulants (Las) or antiphospholipid antibodies (aPLs)
FVIII inhibitors
Various procedures used for detection
No single definitive assay
Unexpected prolonged PT/aPTT
1:1 would be the next step
No correction with PNP
what are the 2 most common inhibitors?
lupus anticoagulant/antiphospholipid antibodies
specific factor inhibitors
lupus anticoagulant (LA) / antiphospholipid anitbodies (aPL)
Originally described as lupus anticoagulants
Now referred to as Antiphospholipid antibody or aPL
Usually IgG
Directed against the protein component of protein-phospholipid complexes
In vivo—thrombotic tendency
In vitro—prolong phospholipid-dependent clotting assays
(lupus inhibitor) LA, LAC, anti-PL
Circulating immunoglobulins (IgG, IgM, or both)
Specific activity against phospholipids
Interferes with phospholipid-dependent complexes that involve factors V & VIII
Tests that may be affected: PT, PTT, DRVVT
More often associated with incidents of thrombosis than hemorrhage
(lupus inhibitor) antiphospholipid antibodies
Seen in a wide variety of autoimmune conditions
Can occur spontaneously
Not all patients with SLE have the inhibitor
Associated with both arterial & venous thromboembolitic events as well as recurrent abortions
These patients RARELY bleed unless there is some additional abnormal hemostasis (ex: prothrombin deficiency or decreased platelets)
(lupus inhibitor) diagnosing LA/aPL
Diagnosis requires demonstration of:
Abnormal PL dependent clotting test
Presence of and inhibitor (clotting test does not correct in mixing study)
Phospholipid-dependent inhibitor
Sample (PPP) integrity important
Procoagulant phospholipids can be in
Patient plasma
Normal plasma used for mixing study
Can neutralize weak lupuslike anticoagulant activities
May give false negative results
lupus anticoagulant vs antiphospholipid screening tests
Two different screening tests recommended
Dilute RVVT (dRVVT)—initial screening test
APTT—second test for screening
If either test is prolonged
Proceed with designated testing
When to suspect a LA or aPL?
If patient presents with a positive history of thrombophilia and APTT is prolonged
Should proceed with testing for LA/aPL
dilute russell’s viper venom test (dRVVT)
Venom from the russell viper (daboia ruesselii)
Reagent
Dilute russell's viper venom, CaCl2, phospholipids (low level)
Added to patient's PPP
Activated FX to produce clot
Used to confirm LA/aPL
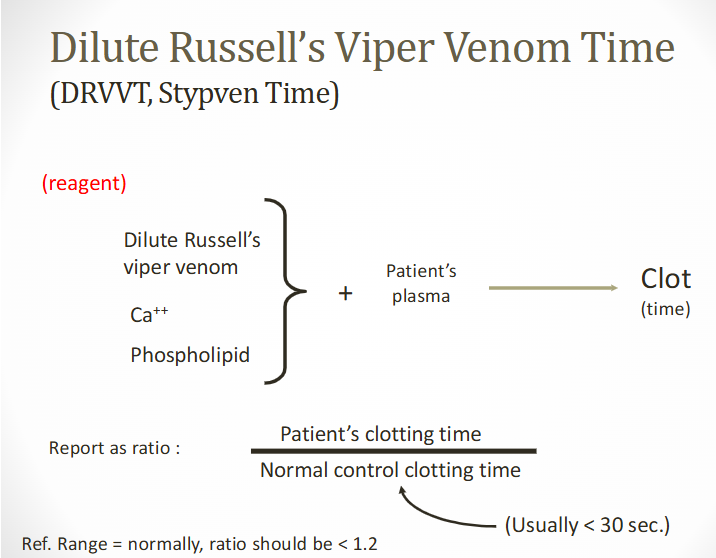
dRVVT ratio & confirmations
Determine ratio of patient's CT to CT of normal control (normal plasma)
LA/aPL is present if patient's dRVVT is longer than normal control
Ratio of patient's CT to normal control CT is <1.2 normally
Confirmatory tests
Use higher PL concentration RVV
Report ratio of high and low PL tests
dilute russell’s viper venom (info)
Has thromboplastic activity & can be substituted for tissue thromboplastin in the PT
Does NOT require Factor VII for activity
Activity is dependent on Factors X, V, prothrombin, fibrinogen
If these factors are present & provide normal levels of activity, the DRVVT is sensitive to lupus anticoagulant
hexagonal phospholipids (HPP)
Substitutes egg phosphatidylethanolamine in a hexagonal phase configuration (HPP)
aPL Abs recognize the HPP configuration
Addition of HPP
Neutralizes inhibitory effect of the aPL antibodies
Does not neutralize factor-specific antibodies
Incubate test plasma with and without HPP
Perform APTT on both mixtures
Test with HPP will have a shortened clotting time if LA or aPL is present
testing algorithm for suspected LA/aPL
Coag screening (PT/PTT)
Mixing study 1:1 (did not correct at initial)
dRVVT or hexagonal PL
Confirm w increasing PL in screening test
(circulating anticoagulants) acquired inhibition of factors
Heparin—most common
VIII—most common specific factor inhibitor
5-20% of hemophilia A patients
IX—5% of hemophilia B patients
Detection of inhibitors is by 1:1 mix using PT or PTT protocol
specific factor inhibiton assay
aka Bethesda titer assay
Developed to measure FVIII inhibitors
Develop in 3-52% of patients with severe hemophilia A
Can be used for other inhibitors to coagulation proteins
Specific Inhibitors
Occur in 10-15% of hemophiliacs at any time after first factor concentrate infusion
Monitor patient's response to treatment by ordering FVIII:C assays
If bleeding does not stop--could be presence of inhibitor
bethesda inhibitor assay (procedure)
Mix patient's plasma with equal volume of PNP of known FVIII activity and incubate for 2 hours
Allows inhibitor to neutralize FVIII in PNP
Perform FVIII assay on incubation mixture to measure residual activity
Convert activity to Bethesda units (BU) using standard Bethesda chart
1BU = 50% residual FVIII
Testing is always done on pre-infusion trough sample
anti-Xa activity assay
Designed to measure plasma heparin (unfractionated & low molecular weight) levels and to monitor anticoagulant therapy
Reference ranges:
Therapeutic ranges of heparin
LMWH: 0.5-1.2 IU/mL
UH: 0.3-0.7 IU/mL
Prophylactic ranges of heparin
LMWH: 0.2-0.5 IU/mL
UH: 0.1-0.4 IU/mL
anti-Xa activity TESTING
When a person is not taking heparin, anti-Xa levels should be zero or undetectable
Activity of both UFH and LMWHs is dependent upon binding to antithrombin (AT)
Binding induces a conformational change in the molecule which accelerates its inhibitory activity
LMWHs have primarily anti-Xa activity while UFH has both anti-Xa and anti-IIa activity
Chromogenic assays are most commonly used