Chemistry Year 11 (copy)
0.0(0)
Card Sorting
1/127
There's no tags or description
Looks like no tags are added yet.
Last updated 8:33 PM on 8/31/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
128 Terms
1
New cards
Mixtures
A mixture is a combination of two or more pure substances (containing one type of molecule) in which each pure substance retains its individual chemical properties - the mixture itself is impure.
2
New cards
Heterogeneous Mixtures
Two or more substances intermingle, but remain physically separate.
.
● Examples include: Dirt+Sand, Oil+Water, Salt+Baking Soda
● A Suspension is a specific type of heterogeneous mixture where particles settle at the bottom
.
● Examples include: Dirt+Sand, Oil+Water, Salt+Baking Soda
● A Suspension is a specific type of heterogeneous mixture where particles settle at the bottom
3
New cards
homgeneous mixture
Two or more substances have merged into a uniform phase. There are no borders between the substances, but they are not chemically bonded. The physical properties of each ingredient can be exploited to separate them.
● Examples include: Saltwater, Copper Sulfate solution
● Saltwater can be distilled (boiled) to separate the water
● The two types include solutions and colloids (particles are present, but are very small and do not settle)
● Examples include: Saltwater, Copper Sulfate solution
● Saltwater can be distilled (boiled) to separate the water
● The two types include solutions and colloids (particles are present, but are very small and do not settle)
4
New cards
physical properties
include magnetism, solubility, density, boiling point, melting point, particle size.
5
New cards
percentage composition
The proportions of each component in a mixture are represented as percentages.
6
New cards
The Periodic Table of Elements
The periodic table is an ordered compilation of all known elements.
7
New cards
Elements vs Compounds
● Elements are pure substances that cannot be chemically or physically decomposed.
● Compounds are pure substances that are chemical combinations of two or more
different elements - they can be decomposed.
● Compounds are pure substances that are chemical combinations of two or more
different elements - they can be decomposed.
8
New cards
Periods
The rows of the periodic table. They increase in atomic number from left-right, and each
period corresponds to the number of electron shells of the elements in that period.
period corresponds to the number of electron shells of the elements in that period.
9
New cards
Groups
The columns of the periodic table. Elements in the same group share similar chemical properties, as they have the same number of valence electrons.
● For example, Group 1 or 7 elements have only one valence electron, so are highly reactive. Group 8 elements have a full shell already, so are highly unreactive as they are already stable.
● For example, Group 1 or 7 elements have only one valence electron, so are highly reactive. Group 8 elements have a full shell already, so are highly unreactive as they are already stable.
10
New cards
Metals, Metalloids and Nonmetals
Uneven chunks of the periodic table that share similar physical properties:
● Metals are are good conductors of heat and electricity, are malleable and ductile, usually have a silvery shine and are usually solid at room temperature.
● Nonmetals are (usually) good insulators of heat and electricity, are brittle; usually dull many of the elemental nonmetals are gases at room temperature, while others are liquids and others are solids.
● Metalloids have properties of both metals and nonmetals, and can be made to conduct electricity in some circumstances.
● Metals are are good conductors of heat and electricity, are malleable and ductile, usually have a silvery shine and are usually solid at room temperature.
● Nonmetals are (usually) good insulators of heat and electricity, are brittle; usually dull many of the elemental nonmetals are gases at room temperature, while others are liquids and others are solids.
● Metalloids have properties of both metals and nonmetals, and can be made to conduct electricity in some circumstances.
11
New cards
three main periodic properties
The three main periodic properties are: Atomic Radius, Ionisation Energy and Electronegativity
12
New cards
Atomic Radius
Half the distance between the centers of two atoms of an element that are touching
● Going left → right across a period, atoms have more protons but the same amount of electron shells. Thus, Electrons are attracted to the nucleus more strongly, and the atomic radius decreases
● Going up → down the group, atoms have more electron shells, which not only put the valence electrons further away, but the inner electrons also repel (or shield) the valence electrons from the nucleus's attraction, so the atomic radius increases
● Cations generally have a smaller ionic radius than the neutral atom, and Anions have a larger atomic radius. This is because ions have a different ratio of protons to electrons, so the radius gets bigger or smaller depending on the electrostatic attraction
NOTE: Atomic radius affects all the other properties - i.e. it's easier for an atom with a greater atomic radius to let go of an electron, because it's valence shell is further away from the nucleus (so greater ionisation energy)
● Going left → right across a period, atoms have more protons but the same amount of electron shells. Thus, Electrons are attracted to the nucleus more strongly, and the atomic radius decreases
● Going up → down the group, atoms have more electron shells, which not only put the valence electrons further away, but the inner electrons also repel (or shield) the valence electrons from the nucleus's attraction, so the atomic radius increases
● Cations generally have a smaller ionic radius than the neutral atom, and Anions have a larger atomic radius. This is because ions have a different ratio of protons to electrons, so the radius gets bigger or smaller depending on the electrostatic attraction
NOTE: Atomic radius affects all the other properties - i.e. it's easier for an atom with a greater atomic radius to let go of an electron, because it's valence shell is further away from the nucleus (so greater ionisation energy)
13
New cards
Ionisation Energy
The energy required to remove one valence electron from a gaseous atom.
● The more strongly bound to the nucleus electrons are, the more ionisation energy is required to remove them
● Smaller atomic radii mean stronger bound electrons, so ionisation energy increases as atomic radius decreases
● A low first ionisation energy indicates that an element is a metal, while a high first ionisation energy indicates that it is a nonmetal
● 1st ionisation energy is the energy required to remove the first electron, while 2nd ionisation energy is the the energy needed to remove the second one, etc
○ Subsequent ionisation energies get higher, because after removing electrons, the ratio of protons to electron becomes skewed to the protons side, and the electrostatic force between them becomes stronger.
○ If there is a full shell after taking out an electron, it requires exponentially more energy to remove the next one from the full shell (because full shells are stable)
● 3s valence orbital has a higher ionisation energy than 3p orbital
● The more strongly bound to the nucleus electrons are, the more ionisation energy is required to remove them
● Smaller atomic radii mean stronger bound electrons, so ionisation energy increases as atomic radius decreases
● A low first ionisation energy indicates that an element is a metal, while a high first ionisation energy indicates that it is a nonmetal
● 1st ionisation energy is the energy required to remove the first electron, while 2nd ionisation energy is the the energy needed to remove the second one, etc
○ Subsequent ionisation energies get higher, because after removing electrons, the ratio of protons to electron becomes skewed to the protons side, and the electrostatic force between them becomes stronger.
○ If there is a full shell after taking out an electron, it requires exponentially more energy to remove the next one from the full shell (because full shells are stable)
● 3s valence orbital has a higher ionisation energy than 3p orbital
14
New cards
How can ionisation equations be represented

15
New cards
electronegativity
The measure of the ability of an atom to attract electrons for chemical bonding (measured in Pauling units)
● When an atom has a smaller atomic radius, it's valence electrons are closer to the nucleus, and the atom can easily pull external electrons into it. Thus, as atomic radius decreases, electronegativity increases
● A high electronegativity difference between atoms indicates a more ionic bond, while a low electronegativity difference indicates a more covalent bond.
● Fluorine is the most electronegative element
● When an atom has a smaller atomic radius, it's valence electrons are closer to the nucleus, and the atom can easily pull external electrons into it. Thus, as atomic radius decreases, electronegativity increases
● A high electronegativity difference between atoms indicates a more ionic bond, while a low electronegativity difference indicates a more covalent bond.
● Fluorine is the most electronegative element
16
New cards
Metallic Character
How close an element is to typical metallic properties - The metallic character of an element is proportional to its ability to lose electrons (i.e. if an element has 1, 2 or 3 valence electrons, it is more metallic than 4, 5, 6, 7 or 8 valence electrons)
17
New cards
Periodic Trends
These properties change moving through the periods (left-right) and groups (up-down):
Moving Left → Right (Periods):
● Ionization Energy Increases
● Electronegativity Increases
● Atomic Radius Decreases
Moving Up → Down (Groups):
● Ionization Energy Decreases
● Electronegativity Decreases
● Atomic Radius Increases
Moving Left → Right (Periods):
● Ionization Energy Increases
● Electronegativity Increases
● Atomic Radius Decreases
Moving Up → Down (Groups):
● Ionization Energy Decreases
● Electronegativity Decreases
● Atomic Radius Increases
18
New cards
Isotopes
While the number of protons defines an element, the number of neutrons indicates the Isotope (different versions) of the element - e.g. a Hydrogen atom can have 0, 1 or 2 neutrons, but it is still hydrogen.
19
New cards
Isotope Stability
The nucleus is held together by a binding energy, and so the ratio of protons to neutrons affects the stability of an isotope.
● Stable Isotopes have sufficient binding energy to keep the nucleus together. They do not undergo radioactive decay
● Unstable Isotopes have an imbalance of neutrons - the binding energy can't hold the nucleus together properly. To become stable, they undergo radioactive decay - and so are also known as radioisotopes
● Stable Isotopes have sufficient binding energy to keep the nucleus together. They do not undergo radioactive decay
● Unstable Isotopes have an imbalance of neutrons - the binding energy can't hold the nucleus together properly. To become stable, they undergo radioactive decay - and so are also known as radioisotopes
20
New cards
Isotope Trends
● Isotopes with atomic number > 82 are all unstable.
● Isotopes with atomic number < 20 and a 1:1 proton-neutron ratio are much more likely
to be stable
● All elements with atomic numbers < 82 have one or more stable isotopes, except for
technetium and promethium
● Atoms with odd numbers of protons and neutrons in the nucleus are more likely to be
unstable
● Atoms with an even number of protons and neutrons are more likely to be stable
● Isotopes with atomic number < 20 and a 1:1 proton-neutron ratio are much more likely
to be stable
● All elements with atomic numbers < 82 have one or more stable isotopes, except for
technetium and promethium
● Atoms with odd numbers of protons and neutrons in the nucleus are more likely to be
unstable
● Atoms with an even number of protons and neutrons are more likely to be stable
21
New cards
Isotope notation
When Isotopes are written as words, the name of the element is given, with a hyphen and number indicating the mass number: For example, helium-3 or carbon-14.
When written as symbols, the chemical symbol is given, with a superscript (mass number) on the upper left, and a subscript (atomic number) on the bottom left.
When written as symbols, the chemical symbol is given, with a superscript (mass number) on the upper left, and a subscript (atomic number) on the bottom left.
22
New cards
Relative atomic mass
The naturally occurring form of an element is usually a mixture of all it's isotopes. The relative atomic masses as given in the periodic table are decimals because they are an average of all the isotopes of that element - dependent on the how common each isotope is.
23
New cards
mass spectrometer
A mass spectrometer is a device that uses electromagnetic fields to sort the isotopes present in a substance by atomic mass, which then allows us to see how abundant each isotope is.
24
New cards
radiation
When an atom undergoes radioactive decay, it is basically breaking apart and releasing energy as particles or waves.
● Neutrons prevent the protons in the nucleus from repelling and breaking away. Atoms decay because the forces holding the nucleus together sometimes aren't strong enough to hold together large nuclei - this occurs when the optimal ratio between protons and neutrons deviate.
● Unstable isotopes/ Radioisotopes undergo radioactive decay. All elements greater than atomic number 92 (Uranium) undergo radioactive decay - these are known as transuranium elements.
● Neutrons prevent the protons in the nucleus from repelling and breaking away. Atoms decay because the forces holding the nucleus together sometimes aren't strong enough to hold together large nuclei - this occurs when the optimal ratio between protons and neutrons deviate.
● Unstable isotopes/ Radioisotopes undergo radioactive decay. All elements greater than atomic number 92 (Uranium) undergo radioactive decay - these are known as transuranium elements.
25
New cards
half life
The half life of a substance is a measure of the time it takes for half the atoms in that substance to decay. Half-lives can range from seconds to billions of years, and can be represented as a logarithmic graph.
26
New cards
three main types of radiation
alpha, beta, gamma
27
New cards
Alpha Decay (Too Much Mass)
Alpha decay (α) occurs when an atom emits an alpha particle - which is made of two protons and two neutrons joined together (a Helium nucleus). This alpha particle is ejected out of the nucleus of the atom.
● Since the number of protons changes, alpha decay causes the atomic mass and element of the atom to change. For example, uranium-238 transforms into thorium-234.
● After Alpha decay, the atomic number decreases by 2, and the mass number decreases by 4
● Since the number of protons changes, alpha decay causes the atomic mass and element of the atom to change. For example, uranium-238 transforms into thorium-234.
● After Alpha decay, the atomic number decreases by 2, and the mass number decreases by 4
28
New cards
Beta Decay (Too Many Neutrons)
Beta Decay (β) occurs when an atom emits a beta particle - which is either an electron or positron (this is known as positron emission). For normal beta decay, an electron is ejected from the nucleus after a neutron splits up into an extra proton and electron.
● Since the neutron turns into a proton, the mass number stays the same, but the atomic number changes. Therefore, beta decay causes an atom to change into another element with the same atomic mass.
● After Beta decay, the atomic number increases by 1, and the mass number stays the same
● Beta Decay also emits a neutrino, but this is negligible
● Since the neutron turns into a proton, the mass number stays the same, but the atomic number changes. Therefore, beta decay causes an atom to change into another element with the same atomic mass.
● After Beta decay, the atomic number increases by 1, and the mass number stays the same
● Beta Decay also emits a neutrino, but this is negligible
29
New cards
Positron Emission
occurs when a proton splits into a neutron and positron (basically a positive electron). It occurs in isotopes that have too many protons.
The atomic number decreases by 1, the mass number stays the same.
The atomic number decreases by 1, the mass number stays the same.
30
New cards
Electron Capture
occurs when a proton captures an electron, and becomes a neutron. The atomic number decreases by 1, and the mass number stays the same.
● A neutrino is emitted for both of the above, but this is negligible.
● A neutrino is emitted for both of the above, but this is negligible.
31
New cards
gamma radiation
Gamma Radiation (γ) occurs when an atom emits gamma rays. It usually occurs after alpha or beta decay, where the nucleus is still excited after decaying. The excited nucleus then releases gamma ray photons to become more stable.
● Since no protons or neutrons are removed/added, the element, atomic number and atomic mass stay the same
● Gamma Radiation technically isn't a type of decay because only energy is released
● Since no protons or neutrons are removed/added, the element, atomic number and atomic mass stay the same
● Gamma Radiation technically isn't a type of decay because only energy is released
32
New cards
Nature of each radiation
Alpha rays:
Helium nucleus
Beta rays:
Electrons
Gamma rays:
Photons
Helium nucleus
Beta rays:
Electrons
Gamma rays:
Photons
33
New cards
Penetrative power of each radiation
Alpha: Few centimetres in air
Beta: Few millimetres of aluminium
Gamma: Many centimetres of lead
Beta: Few millimetres of aluminium
Gamma: Many centimetres of lead
34
New cards
Charge of each radiation
Alpha: +2e
Beta: -e
Gamma: Zero
Beta: -e
Gamma: Zero
35
New cards
Mass of each radiation
Alpha: 6.64 x 10- 27 Kg
Beta: 9.1 x 10- 31 Kg
Gamma: Zero
Beta: 9.1 x 10- 31 Kg
Gamma: Zero
36
New cards
Bohr Model: main points
- expanded on Rutherford's, but defined fixed energy levels
- Niels Bohr developed a new model of the atom in 1913 called the "planetary model"
His model had four main points (postulates):
1. Electrons are particles that occupy fixed orbits around the nucleus - called stationary orbits
2. Each orbit has an energy level associated with it
3. Energy is absorbed when an electron jumps from a lower orbit to a higher one and
energy is emitted when an electron falls from a higher to a lower orbit
○ The energy and frequency of light emitted can be calculated using the
difference between the two orbital energy levels
4. Electrons cannot exist between the energy levels/orbits
- Niels Bohr developed a new model of the atom in 1913 called the "planetary model"
His model had four main points (postulates):
1. Electrons are particles that occupy fixed orbits around the nucleus - called stationary orbits
2. Each orbit has an energy level associated with it
3. Energy is absorbed when an electron jumps from a lower orbit to a higher one and
energy is emitted when an electron falls from a higher to a lower orbit
○ The energy and frequency of light emitted can be calculated using the
difference between the two orbital energy levels
4. Electrons cannot exist between the energy levels/orbits
37
New cards
Bohr model supporting evidence and limitations
The flame test supports Bohr's model - the different lights being emitted from the flame test indicated that energy was emitted when electrons returned to their ground shells, and the single spectral lines indicated that there were set routes/shells for the electrons to follow.
However, Bohr's model had limitations:
● It could only be applied to atoms with one valence electron
● It didn't explain the different intensities of lines/colours in a hydrogen spectrum
● It didn't explain the Zeeman effect (idek)
However, Bohr's model had limitations:
● It could only be applied to atoms with one valence electron
● It didn't explain the different intensities of lines/colours in a hydrogen spectrum
● It didn't explain the Zeeman effect (idek)
38
New cards
Schrodinger's Model
(Quantum Mechanical Model)
- Bohr believed that electrons orbited the nucleus in a fixed radius from the nucleus
Schrodinger proposed that they moved around a lot more irregularly, and didn't completely follow strict paths - this explained the issues of Bohr's model.
● Schrodingers model was based on the wave-particle duality of electrons.
- In 1926, Schrodinger developed the quantum mechanical model of the atom, which predicts the probability of an electron being at a certain location around the atom instead of defining a set radius - basically, proposing that electrons do not travel in fixed radii, but in a particular area/tubes around the atom.
It was modeled as a nucleus surrounded by an "electron cloud". Electrons are most likely to be found where the cloud is most dense.
- Bohr believed that electrons orbited the nucleus in a fixed radius from the nucleus
Schrodinger proposed that they moved around a lot more irregularly, and didn't completely follow strict paths - this explained the issues of Bohr's model.
● Schrodingers model was based on the wave-particle duality of electrons.
- In 1926, Schrodinger developed the quantum mechanical model of the atom, which predicts the probability of an electron being at a certain location around the atom instead of defining a set radius - basically, proposing that electrons do not travel in fixed radii, but in a particular area/tubes around the atom.
It was modeled as a nucleus surrounded by an "electron cloud". Electrons are most likely to be found where the cloud is most dense.
39
New cards
Energy Levels and Electron Configuration
Electrons do not orbit the nucleus in fixed orbits, they move around in energy levels called
orbitals - which are regions around the atom where the electron is very likely to be found at any given time.
orbitals - which are regions around the atom where the electron is very likely to be found at any given time.
40
New cards
Aufbau Principle
The Aufbau Principle states that in order to configure electrons in an atom, electrons are added to the lowest energy level until it is filled. Then electrons are added to the next energy level until that is filled, etc.
41
New cards
Electron Subshells
Each electron shell is actually composed of various subshells with different capacities - just like a road is composed of different sized lanes.
● Each subshell is a group of atomic orbitals - the orbitals that make up each subshell have distinct shapes, and each orbital can hold 2 electrons
Orbitals come in groups - There is always one s orbital, three p orbitals, five d orbitals and so on.
● I.e. even though the d subshell in the 3rd principal shell is called 3d, there are Five d orbitals in it.
● Each subshell is a group of atomic orbitals - the orbitals that make up each subshell have distinct shapes, and each orbital can hold 2 electrons
Orbitals come in groups - There is always one s orbital, three p orbitals, five d orbitals and so on.
● I.e. even though the d subshell in the 3rd principal shell is called 3d, there are Five d orbitals in it.
42
New cards
Principal Shell → Subshells → Orbitals
The principal shells are named in increasing order of energy as 1,2,3, etc, while the subshells are named s, p, d, f, g. Each subshell can hold a maximum amount of electrons, depending on the orbitals that it contains.
43
New cards
- Subshell
- No. and type of Orbitals
- Electron capacity
- No. and type of Orbitals
- Electron capacity
- Subshell
- No. and type of Orbitals
- Electron capacity
s
One s orbital
2
p
Three p orbitals
6
d
Five d orbitals
10
f
Seven f orbitals
14
g
Nine g orbitals
18
- No. and type of Orbitals
- Electron capacity
s
One s orbital
2
p
Three p orbitals
6
d
Five d orbitals
10
f
Seven f orbitals
14
g
Nine g orbitals
18
44
New cards
Blocks of the Periodic Table
The periodic table can also be divided into four blocks - s-block, p-block, d-block and f-block, corresponding to the orbitals. I.e. s-block elements all have their valence electrons in the s-orbital, p-block elements all have their valence electrons in the p-orbital, etc.
45
New cards
Flame Test
The flame test is an analytical experiment used to detect the presence of particular metals in a sample. When heated, the electrons in a metal atom become excited, and move to higher energy levels. As they return to their normal energy levels, they release the excess energy as photons (light).
The colour of the light (frequency of photon) depends on the energy gap between the energy levels - thus, the colour of the flame indicates which metal it is, as different metals have different energy gaps between energy levels:
The colour of the light (frequency of photon) depends on the energy gap between the energy levels - thus, the colour of the flame indicates which metal it is, as different metals have different energy gaps between energy levels:
46
New cards
mass spectrometer
A device called a mass spectrometer splits the colours into much more specific orders, displaying these colours in an emission spectrum - which is unique to each element, like its fingerprint. This enables more precise identification of elements.
47
New cards
Flame test colours
For example, copper produces a blue flame, lithium and strontium a red flame, calcium an orange flame, sodium a yellow flame, and barium a green flame.
48
New cards
Bonding
When an atom has too little/too many electrons in the valence shell, it wants to remove/gain electrons to make it stable (full valence shell). Thus, when it makes contact with another element that has the amount of electrons that it needs, they bond together.
This concept is related to Electronegativity, and is the main mechanism behind bonding.
This concept is related to Electronegativity, and is the main mechanism behind bonding.
49
New cards
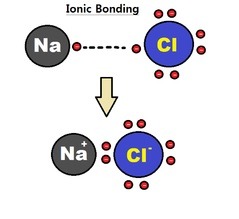
Ionic Bonds (Metal + Nonmetal)
Ionic Bonds involve a Metal donating electrons to a Nonmetal atom
● The metal atom becomes positively charged (cation) after losing electrons
● The nonmetal atom becomes negatively charged (anion) after gaining electrons
● The oppositely-charged atoms (cation (+) and anion (-)) attract to create an ionic
compound
For example, when Sodium bonds with Chlorine - Sodium donates its one valence electron to Chlorine, making Sodium positive (cation), and Chlorine negative (anion). They attract and bond to form Sodium Chloride.
● The metal atom becomes positively charged (cation) after losing electrons
● The nonmetal atom becomes negatively charged (anion) after gaining electrons
● The oppositely-charged atoms (cation (+) and anion (-)) attract to create an ionic
compound
For example, when Sodium bonds with Chlorine - Sodium donates its one valence electron to Chlorine, making Sodium positive (cation), and Chlorine negative (anion). They attract and bond to form Sodium Chloride.
50
New cards
Polyatomic Ions
Polyatomic ions are molecules that are composed of bonded atoms, but still have an overall
charge. Examples include Nitrate, (NO3- ), Nitrite (NO2- ) and Ammonium (NH4+ ).
charge. Examples include Nitrate, (NO3- ), Nitrite (NO2- ) and Ammonium (NH4+ ).
51
New cards
Covalent Bonds (Non-metal + Non-metal)
Covalent Bonds involve a Nonmetal sharing electrons with another Nonmetal atom
● The atom with excess valence electrons shares them with the atom that needs them
● They bond as these electrons are shared to form a covalent or molecular compound
For example, when Hydrogen bonds with Oxygen, Two hydrogen atoms each share an electron with an Oxygen atom and form Water (H2O ).
● The atom with excess valence electrons shares them with the atom that needs them
● They bond as these electrons are shared to form a covalent or molecular compound
For example, when Hydrogen bonds with Oxygen, Two hydrogen atoms each share an electron with an Oxygen atom and form Water (H2O ).
52
New cards
Polarity of Covalent Bonds
Although electrons are shared in covalent bonds, they are not always shared equally. This occurs when one atom has higher electronegativity (i.e. wants the electrons more) than the other:
In Non-Polar Covalent Bonds, Electrons are shared equally between the atoms, because the electronegativity of the atoms is the same or very similar (
In Non-Polar Covalent Bonds, Electrons are shared equally between the atoms, because the electronegativity of the atoms is the same or very similar (
53
New cards
The higher the electronegativity difference, the more _______ the bond is
ionic the bond is.
● The electronegativity of an element can generally be predicted by its position in the periodic table (periodicity) - so two elements such as Hydrogen and Bromine which are located far from each other would make a very polar covalent bond.
● The electronegativity of an element can generally be predicted by its position in the periodic table (periodicity) - so two elements such as Hydrogen and Bromine which are located far from each other would make a very polar covalent bond.
54
New cards
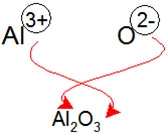
criss cross method
To easily determine the final product of an ionic bond (or some polar covalent bonds), you can find the two reactants and their oxidation numbers, then swap the numbers and make them the subscripts for the product.
This ensures that the ratios of ions are correct - you need two Aluminium atoms for every three Oxygen atoms to make aluminium oxide (total charges of +6 and -6 cancel out)
If you have something like Sn2O 4 after crisscrossing, you can simplify that to most basic ratio (1 Sn: 2 O) using it's highest common factor and write it as SnO2
However, you can criss cross but not simplify polyatomic ions - e.g. Na2S O4 can't be simplified
This ensures that the ratios of ions are correct - you need two Aluminium atoms for every three Oxygen atoms to make aluminium oxide (total charges of +6 and -6 cancel out)
If you have something like Sn2O 4 after crisscrossing, you can simplify that to most basic ratio (1 Sn: 2 O) using it's highest common factor and write it as SnO2
However, you can criss cross but not simplify polyatomic ions - e.g. Na2S O4 can't be simplified
55
New cards
Oxidation Numbers
The oxidation number is the charge an atom would have if it were an ion (charged atom). To
clarify:
Atoms want to remove electrons when they have 1, 2, or 3 valence electrons, and want to gain electrons when they have 4, 5, 6 or 7 valence electrons.
● When an atom wants to get rid of electrons, it has a positive oxidation number. For example, Sodium has one valence electron, so it has an oxidation number of +1. Aluminium has 3 valence electrons, so has an oxidation number of +3.
● When an atom wants to gain electrons, it has a negative oxidation number. For example, Fluorine wants to gain one electron (it has 7 valence electrons), so has an oxidation number of -1.
The amount of valence electrons (oxidation number) dictates the ratio of atoms in a bond. For example - with Potassium +1 and Oxygen -2, two atoms of Potassium are needed for every one oxygen atom. Thus, the chemical formula for Potassium Oxide is K2O
clarify:
Atoms want to remove electrons when they have 1, 2, or 3 valence electrons, and want to gain electrons when they have 4, 5, 6 or 7 valence electrons.
● When an atom wants to get rid of electrons, it has a positive oxidation number. For example, Sodium has one valence electron, so it has an oxidation number of +1. Aluminium has 3 valence electrons, so has an oxidation number of +3.
● When an atom wants to gain electrons, it has a negative oxidation number. For example, Fluorine wants to gain one electron (it has 7 valence electrons), so has an oxidation number of -1.
The amount of valence electrons (oxidation number) dictates the ratio of atoms in a bond. For example - with Potassium +1 and Oxygen -2, two atoms of Potassium are needed for every one oxygen atom. Thus, the chemical formula for Potassium Oxide is K2O
56
New cards
Lewis Dot Diagrams
Lewis Dot Diagrams are a form of shorthand notation for covalent compounds, showing their valence electrons and bonds.
● Atoms are represented by the element symbol
● Valence Electrons are represented as dots and are placed in pairs around the atom
● Covalent bonds with other atoms are represented as lines
● Atoms are represented by the element symbol
● Valence Electrons are represented as dots and are placed in pairs around the atom
● Covalent bonds with other atoms are represented as lines
57
New cards
To draw a Lewis dot diagram for CO2
1. Find the number of valence electrons of each element in the compound.
● In this case, Carbon has 4 valence electrons, and Oxygen has 6
2. Place the atom with the lowest electronegativity in the centre, then place the other
atoms around it.
● In this case, carbon is in the centre as it is the least electronegative
O C O
3. Arrange the electrons so that each atom contributes one electron to a bond (a single bond is made of two electrons from separate atoms)
If all the valence shells are now stable (i.e. have 8 electrons), then the Lewis diagram is done.
● However, in this case, Oxygen has 7 valence electrons (six + the one carbon shared) each while carbon has 6 (4 + the two both oxygens shared)
4. If the valence shells are incomplete, then add another electron to make a double bond
Step 4 should be repeated until the valence shells are stable. In this case, the valence shells of oxygen and carbon both have 8 electrons, so it is stable.
5. Arrange the electrons into pairs
The pairs of electrons that are not bonded are called - non-bonding electron pairs.
● In this case, Carbon has 4 valence electrons, and Oxygen has 6
2. Place the atom with the lowest electronegativity in the centre, then place the other
atoms around it.
● In this case, carbon is in the centre as it is the least electronegative
O C O
3. Arrange the electrons so that each atom contributes one electron to a bond (a single bond is made of two electrons from separate atoms)
If all the valence shells are now stable (i.e. have 8 electrons), then the Lewis diagram is done.
● However, in this case, Oxygen has 7 valence electrons (six + the one carbon shared) each while carbon has 6 (4 + the two both oxygens shared)
4. If the valence shells are incomplete, then add another electron to make a double bond
Step 4 should be repeated until the valence shells are stable. In this case, the valence shells of oxygen and carbon both have 8 electrons, so it is stable.
5. Arrange the electrons into pairs
The pairs of electrons that are not bonded are called - non-bonding electron pairs.
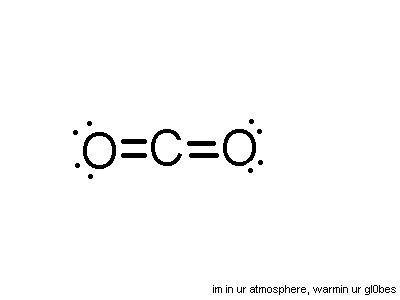
58
New cards
Lewis Structures for Ionic compounds
In an ionic bond, one atom becomes a cation, and the other becomes an anion. This is represented in Lewis diagrams by the cation having a positive superscript, and the anion having a negative superscript, with square brackets around it.
59
New cards
Intramolecular bonds
are the bonds that hold atoms together within a molecule.
60
New cards
intermolecular bonds
are the forces that exist between molecules.
61
New cards
are intramolecular bonds stronger than intermolecular forces?
Intramolecular bonds are generally much stronger than Intermolecular forces - breaking apart the atoms inside a molecule requires a lot more energy than breaking molecules away from each other.
While intramolecular forces always arise from chemical bonds, intermolecular forces can also arise from electromagnetic attractions holding molecules together.
While intramolecular forces always arise from chemical bonds, intermolecular forces can also arise from electromagnetic attractions holding molecules together.
62
New cards
Intramolecular bonds types
Types of intramolecular bonds include ionic, covalent and metallic bonds.
63
New cards
The more electrons an atom has (i.e larger atomic radius), the ... its intermolecular forces
The more electrons an atom has (i.e larger atomic radius), the stronger it's intermolecular forces. The state of matter at room temperature can be used to compare the strength of the intermolecular forces between two elements/compounds - if it is a solid, it has stronger intermolecular forces than a substance that is a gas at room temp.
64
New cards
Dipole-Dipole Interactions
When multiple molecules are in polar covalent or ionic bonds, one side of the molecule is slightly positive, and one side is slightly negative. These sides are known as dipoles. When the negative dipole of one molecule attracts to the positive dipole of another, they bond. This is the strongest intermolecular force.

65
New cards
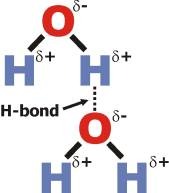
Hydrogen Bonds
a special kind of dipole-dipole interaction that occurs between hydrogen atoms bonded to either oxygen, nitrogen, or fluorine atoms. The positive dipole of Hydrogen is attracted to the negative dipole of oxygen/nitrogen/fluorine. Due to the large electronegativity difference, this is the strongest version of dipole-dipole interactions.
66
New cards
Dispersion Forces
are the weakest intermolecular force. They intrinsically exist between all molecules (doesn't have to be ionic/polar), and occur due to the movement of electrons forming temporary dipoles. The more electrons a molecule has, the stronger the Van der Waal forces.
67
New cards
Physical Properties of Elements/Compounds due to Intra/Intermolecular forces
Polar covalent/Ionic compounds have strong dipole-dipole interactions between them, while Nonpolar compounds have only weak London dispersion forces. The stronger a bond is, the more energy is required to break it, so ionic and polar compounds tend to have higher melting and boiling points than nonpolar compounds.
For example, methane boils at -161.5°C, while water, a polar covalent compound, boils at 100°C. An ionic compound such as sodium chloride has a much higher boiling point, at 1413°C.
For example, methane boils at -161.5°C, while water, a polar covalent compound, boils at 100°C. An ionic compound such as sodium chloride has a much higher boiling point, at 1413°C.
68
New cards
four main types of chemical structures
The four main types of chemical structures are: Ionic network, Covalent network, Covalent Molecular and Metallic.
69
New cards
Ionic Network
Ionic Compounds form lattice structures, where each cation is surrounded by 6 anions, and each anion is surrounded by 6 cations - forming something like a cube like NaCl does
● Although one Sodium atom only gives an electron to one Chlorine, the attraction is equal in all directions, so the bond is non-directional.
○ This makes the bonds very strong, so ionic compounds have high melting and boiling points
● A single molecule of an ionic compound cannot exist, they always exist in lattices - so they are referred to as Formula Units instead of molecules, and are always in the simplest ratio
There are two methods of electrical conductivity - movement of electrons, or the movement of ions.
● Ionic solids have poor electrical conductivity - they have no free electrons, because they are locked into the oppositely charged atoms, and the ions are stuck in a lattice structure.
● Molten ionic substances have good electrical conductivity - the application of heat energy breaks the lattice, so the ions can move more and conduct electricity
● Aqueous ionic substances have extremely good electrical conductivity, as the solid dissociates into its ions, they have a large and easy range of movement to conduct electricity
Ionic solids are brittle (they break upon impact).
When an impact displaces some of the lattice layers, two or more of the same ion may align. Since they have the same charge, the ions that are in line repel each other, and the solid breaks.
● Although one Sodium atom only gives an electron to one Chlorine, the attraction is equal in all directions, so the bond is non-directional.
○ This makes the bonds very strong, so ionic compounds have high melting and boiling points
● A single molecule of an ionic compound cannot exist, they always exist in lattices - so they are referred to as Formula Units instead of molecules, and are always in the simplest ratio
There are two methods of electrical conductivity - movement of electrons, or the movement of ions.
● Ionic solids have poor electrical conductivity - they have no free electrons, because they are locked into the oppositely charged atoms, and the ions are stuck in a lattice structure.
● Molten ionic substances have good electrical conductivity - the application of heat energy breaks the lattice, so the ions can move more and conduct electricity
● Aqueous ionic substances have extremely good electrical conductivity, as the solid dissociates into its ions, they have a large and easy range of movement to conduct electricity
Ionic solids are brittle (they break upon impact).
When an impact displaces some of the lattice layers, two or more of the same ion may align. Since they have the same charge, the ions that are in line repel each other, and the solid breaks.
70
New cards
Covalent molecular
Sometimes, Covalent compounds exist as discrete molecules, with weak intermolecular forces between each molecule.
Depending on the strength of the intermolecular forces, covalent molecular compounds can
be liquids, solids or gases - thus, they have a wide range of melting and boiling points
Covalent Molecular substances do not conduct electricity because there are no ions or free floating electrons
Depending on the strength of the intermolecular forces, covalent molecular compounds can
be liquids, solids or gases - thus, they have a wide range of melting and boiling points
Covalent Molecular substances do not conduct electricity because there are no ions or free floating electrons
71
New cards
metallic
A lattice of positive metal ions are held together by delocalised electrons (electron cloud/ electron sea) in metallic lattices.
● The delocalised electrons carry electricity very well, making metals great conductors of electricity.
● They also carry heat energy in the form of kinetic energy by moving around and hitting the colder parts of the lattice, making metals great conductors of heat
● Delocalised electrons are also responsible for the malleability/ductility of metals -
when metal ions are displaced, the electron cloud acts as a 'glue' and stops the ions from repelling and breaking apart
● The delocalised electrons carry electricity very well, making metals great conductors of electricity.
● They also carry heat energy in the form of kinetic energy by moving around and hitting the colder parts of the lattice, making metals great conductors of heat
● Delocalised electrons are also responsible for the malleability/ductility of metals -
when metal ions are displaced, the electron cloud acts as a 'glue' and stops the ions from repelling and breaking apart
72
New cards
Allotropes
Allotropes are different structures of the same element - due to their different structures, they have different physical and chemical properties - i.e. diamond vs carbon as above. Sometimes, an allotrope of an element may not have a structure - this is known as amorphous. An example is carbon as soot.
73
New cards
Chemical Shapes (Molecular Geometry)
Different molecules form different shapes depending on their composition. The main types of molecular shapes are:
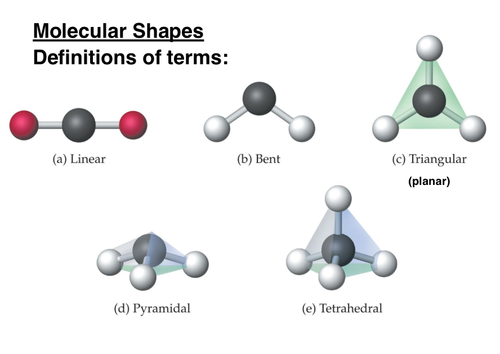
74
New cards
Valence shell electron pair repulsion (VSEPR) theory CO2 AND H2O
The shape of a molecule depends on the electron configurations of its atoms - known as Valence shell electron pair repulsion (VSEPR) theory.
● For example, CO2 has its nonbonding electrons distributed equally on opposite sides (it is nonpolar), so has a linear structure, since the repulsion is equal on either side.
● H2O has it's nonbonding electrons on top of oxygen, so they repel the hydrogen atoms downwards. As the hydrogen atoms come closer together, they also repel each other, creating a bent molecular shape
● For example, CO2 has its nonbonding electrons distributed equally on opposite sides (it is nonpolar), so has a linear structure, since the repulsion is equal on either side.
● H2O has it's nonbonding electrons on top of oxygen, so they repel the hydrogen atoms downwards. As the hydrogen atoms come closer together, they also repel each other, creating a bent molecular shape
75
New cards
Naming Covalent (Molecular) Compounds
● Name the element with lowest electronegativity first
● Add the appropriate suffix to the second element (-ide)
● Add a prefix to both the first and second element to indicate the number of atoms
○ I.e. mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca
○ Don't add the mono prefix to the first element
● For example, Carbon Dioxide or Dichlorine Monoxide
● Add the appropriate suffix to the second element (-ide)
● Add a prefix to both the first and second element to indicate the number of atoms
○ I.e. mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca
○ Don't add the mono prefix to the first element
● For example, Carbon Dioxide or Dichlorine Monoxide
76
New cards
Naming Ionic Compounds
● Name the cation (metal) first, then the anion (nonmetal) second
● Add the appropriate suffix to the anion (-ide, -ate, -ite)
● For example. NaCl is Sodium Chloride, AlO2 is Aluminium Oxide, etc
● Add the appropriate suffix to the anion (-ide, -ate, -ite)
● For example. NaCl is Sodium Chloride, AlO2 is Aluminium Oxide, etc
77
New cards
Stoichiometry
Since the Law of Conservation of Mass states that matter cannot be created or destroyed, the atoms before and after the reaction must be the same.
Thus, Chemical Equations can be balanced by adding stoichiometric coefficients in front of the molecules.
E.g.2Na(s)+Cl2 (g)→2NaCl(s)
This means that for every two separate Sodium atoms (total mass: 45.98 amu) and diatomic Chlorine molecule (70.90 amu) that react, 2 molecules worth (275.9 amu) of Sodium Chloride is produced.
Thus, Chemical Equations can be balanced by adding stoichiometric coefficients in front of the molecules.
E.g.2Na(s)+Cl2 (g)→2NaCl(s)
This means that for every two separate Sodium atoms (total mass: 45.98 amu) and diatomic Chlorine molecule (70.90 amu) that react, 2 molecules worth (275.9 amu) of Sodium Chloride is produced.
78
New cards
Moles
A mole is a unit of measurement, defined as 6.022 x 10^23 (Avogadro's Constant) particles of a compound/element, with the symbol n.
● E.g. 1 Mole of Sodium is equal to 6.022 x 102 3 Sodium atoms
This system of measurement is relative to the Carbon-12 atom (1 mole of carbon 12 weighs exactly 12 grams)
● E.g. 1 Mole of Sodium is equal to 6.022 x 102 3 Sodium atoms
This system of measurement is relative to the Carbon-12 atom (1 mole of carbon 12 weighs exactly 12 grams)
79
New cards
Molar Mass
The mass in grams of one mole of a molecule is known as the Molar Mass of the molecule, measured in grams per mole (g/mol). The Molar Mass of a molecule is equal to its atomic mass but in grams.
● E.g. Sodium (atomic mass: 22.99 amu) has a molar mass of 22.99 g/mol
The relationship between Moles, Actual Mass and Molar mass is given by the formula:
n = m/MM
Number of Moles = Mass(g) ÷ Molar Mass(g/mol)
● E.g. Sodium (atomic mass: 22.99 amu) has a molar mass of 22.99 g/mol
The relationship between Moles, Actual Mass and Molar mass is given by the formula:
n = m/MM
Number of Moles = Mass(g) ÷ Molar Mass(g/mol)
80
New cards
Other mole formulae
(Number of Moles = Number of Particles / Avogadros Number)
n = CV
(Number of Moles = Concentration of Solution (moles/litre) * Volume (litres))
n = Volume of Gas / 22.71 OR 24.79 (STP or RTP)
n = CV
(Number of Moles = Concentration of Solution (moles/litre) * Volume (litres))
n = Volume of Gas / 22.71 OR 24.79 (STP or RTP)
81
New cards
Molar Stoichiometry
To describe the Sodium + Chlorine -> Sodium Chloride reaction in molar terms, it would be 2 moles of Na reacting with 1 Mole of Cl2 to produce 2 Moles of NaCl.
These coefficients may not always be integers - you may need to calculate How much product is produced if 0.5 Moles of Chlorine is reacted.
To do this, find the ratio between the reactant and product in the balanced equation, and use your maths skills:
1Cl:2NaCl →1/2→0.5/x
x = 1 ( 1 mole of NaCl is produced)
This concept can also be applied to reactant vs reactant. The ratio between two reactants/ reactant and product is known as the stoichiometric ratio.
ALWAYS CONVERT WEIGHT ETC TO MOLES BEFORE DOING CALCULATIONS
These coefficients may not always be integers - you may need to calculate How much product is produced if 0.5 Moles of Chlorine is reacted.
To do this, find the ratio between the reactant and product in the balanced equation, and use your maths skills:
1Cl:2NaCl →1/2→0.5/x
x = 1 ( 1 mole of NaCl is produced)
This concept can also be applied to reactant vs reactant. The ratio between two reactants/ reactant and product is known as the stoichiometric ratio.
ALWAYS CONVERT WEIGHT ETC TO MOLES BEFORE DOING CALCULATIONS
82
New cards
Limiting reagents
The limiting reagent in a chemical reaction is the substance that is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent, since the reaction cannot continue without it, and will leave an excess of another substance.
83
New cards
Concentration and Molarity
Concentration refers to the amount of solute divided by the volume of solvent. It can be measured in g/L, n/L, g/kg, mL/L, ppm (parts per million).
● Colour is a good indicator of how concentrated a solution is, since the more solute is added, the darker the colour gets.
● Molarity is a unit of concentration, defined as moles of solute per litre of solvent (n/L).
n =CxV
C1V1 = C2V2
*Convert concentrations to mol/L if required in calculations
● Colour is a good indicator of how concentrated a solution is, since the more solute is added, the darker the colour gets.
● Molarity is a unit of concentration, defined as moles of solute per litre of solvent (n/L).
n =CxV
C1V1 = C2V2
*Convert concentrations to mol/L if required in calculations
84
New cards
Avogadro's Gas Law
When measured at the same temperature and pressure, equal volumes of different gases contain the same number of molecules.
V∝n
(Volume is proportional to number of Moles)
E.g.
V1/ n1 = V2/ n2 N2 + 3H2 → 2NH3
If Nitrogen was 100ml, then 300ml of H2 is needed for 200ml of Ammonia H2 + Cl2 → 2HCl
100 mL Hydrogen gas reacts with 100 mL Chlorine gas to produce 200ml of Hydrogen Chloride gas
V∝n
(Volume is proportional to number of Moles)
E.g.
V1/ n1 = V2/ n2 N2 + 3H2 → 2NH3
If Nitrogen was 100ml, then 300ml of H2 is needed for 200ml of Ammonia H2 + Cl2 → 2HCl
100 mL Hydrogen gas reacts with 100 mL Chlorine gas to produce 200ml of Hydrogen Chloride gas
85
New cards
Kelvin to celsius
add 273.15 to ur temperature thats in celsius
86
New cards
Standard Solutions
A standard solution is a solution of known and fixed concentration. A known weight of solute is dissolved to make a specific volume.
Primary standards are used to prep them - a primary standard is a reagent which can be weighed easily, and which is so pure that its weight is truly representative of the number of moles of substance contained. Not all substances can be used as primary standards.
● Sodium Hydroxide absorbs water rapidly, so it's difficult to know the actual mass The properties of a good primary standard include: high purity, low reactivity, non-toxic, not
likely to absorb moisture, high equivalent weight
How to make a Standard Solution:
1. Weigh the primary standard - the (pure) solid dissolved to form the standard solution.
2. Dissolve the primary standard in a small amount of distilled water in a beaker.
3. Pour the contents of the beaker into a volumetric flask, rinsing the beaker with
slightly more distilled water and pouring in to ensure all contents are transferred.
4. Add distilled water to the flask up to the calibration point. Use a graduated pipette to
deliver the final amount.
Each step in the procedure is designed to increase accuracy and precision as much as possible, to ensure that concentration of the solution is accurate and precise.
Primary standards are used to prep them - a primary standard is a reagent which can be weighed easily, and which is so pure that its weight is truly representative of the number of moles of substance contained. Not all substances can be used as primary standards.
● Sodium Hydroxide absorbs water rapidly, so it's difficult to know the actual mass The properties of a good primary standard include: high purity, low reactivity, non-toxic, not
likely to absorb moisture, high equivalent weight
How to make a Standard Solution:
1. Weigh the primary standard - the (pure) solid dissolved to form the standard solution.
2. Dissolve the primary standard in a small amount of distilled water in a beaker.
3. Pour the contents of the beaker into a volumetric flask, rinsing the beaker with
slightly more distilled water and pouring in to ensure all contents are transferred.
4. Add distilled water to the flask up to the calibration point. Use a graduated pipette to
deliver the final amount.
Each step in the procedure is designed to increase accuracy and precision as much as possible, to ensure that concentration of the solution is accurate and precise.
87
New cards
Chemical Reactions
Chemical reaction occur when Chemical Bonds are broken, rearranged and established to form new substances. They involve reactants turning into products.
88
New cards
Indicators of a chemical reaction include:
1. Bubbles
2. Colour change
3. Change in Energy (change in temperature)
4. Appearance of a solid (due to precipitation)
5. Disappearance of a solid (not due to dissolving)
6. New Substance ← this is the only thing that guarantees a chemical reaction occurring,
the rest are indicators
2. Colour change
3. Change in Energy (change in temperature)
4. Appearance of a solid (due to precipitation)
5. Disappearance of a solid (not due to dissolving)
6. New Substance ← this is the only thing that guarantees a chemical reaction occurring,
the rest are indicators
89
New cards
Exothermic reaction
The reactants have more energy than the products
● This energy is released from bonds being formed and goes into the surroundings,
usually causing the temperature to rise
● Examples include most Combustion reactions
● This energy is released from bonds being formed and goes into the surroundings,
usually causing the temperature to rise
● Examples include most Combustion reactions
90
New cards
Endothermic reaction and what else can be endothermic or exothermic
energy is taken from the surroundings and the reactants have less energy than the products
● Because energy is being used to break bonds, endothermic reactions usually cause the temperature to drop
● Examples include most Decomposition reactions
● Because energy is being used to break bonds, endothermic reactions usually cause the temperature to drop
● Examples include most Decomposition reactions
91
New cards
Synthesis is the formation of compounds by combining simpler substances such as elements
● Synthesis reactions are exothermic (energy is given off when bonds are broken)
● Examples of synthesis reactions include:
○ 2Na + Cl2 → 2NaCl
● Synthesis reactions are exothermic (energy is given off when bonds are broken)
● Examples of synthesis reactions include:
○ 2Na + Cl2 → 2NaCl
Synthesis Reactions (A + B → AB)
92
New cards
When salts dissolve in water, the bonds between the cation and anion separate inside the solution
● When two of these solutions containing soluble salts (that have unpaired cations and anions) are mixed, the cation-anion pairings sometimes swap creating new salts
○ This swapping of ions when two salt solutions are mixed is called displacement
○ Both reactants have to be soluble
○ An example displacement reaction is: Na2S + 2HCl → 2NaCl + H2S
● When two of these solutions containing soluble salts (that have unpaired cations and anions) are mixed, the cation-anion pairings sometimes swap creating new salts
○ This swapping of ions when two salt solutions are mixed is called displacement
○ Both reactants have to be soluble
○ An example displacement reaction is: Na2S + 2HCl → 2NaCl + H2S
Displacement Reactions (A + BC → AC + B or AB + CD → AD + CB)
93
New cards
After displacement, sometimes one of the new salts is insoluble and falls to the bottom if heavy enough. This settling of the heavy insoluble salt is called precipitation, and the solid particles formed are called the precipitate
● An example of this is: AgNO3 + NaCl → AgCl + NaNO3
● AgCl (silver chloride) is insoluble, so forms a white precipitate
Whether or not such a reaction occurs can be determined using the solubility rules
● An example of this is: AgNO3 + NaCl → AgCl + NaNO3
● AgCl (silver chloride) is insoluble, so forms a white precipitate
Whether or not such a reaction occurs can be determined using the solubility rules
Precipitation Reactions (Soluble Salt A (aq) + Soluble Salt B (aq) → Precipitate (s) + Soluble Salt C (aq))
94
New cards
● In decomposition, the atoms of a compound are separated to form two or more products
Decomposition Reactions (AB → A + B)
95
New cards
● Thermal
○ Decomposed through the application of heat, making it an endothermic
reaction
○ E.g. CaCO3 → CaO + CO2
■ Calcium carbonate → Calcium Oxide + Carbon Dioxide gas
● Electrolytic
○ Decomposition due to an electric current being passed through an aqueous
solution of a compound
■ Electrolytic cells are used for electrolytic decomposition
○ E.g.NaCl→Na+Cl
■ Sodium Chloride → Sodium + Chloride
○ E.g. 2H2O → 2H2 + O2
■ Water → Hydrogen gas + Oxygen gas
● Photo Decomposition
○ Decomposition due to use of light energy/photons
○ E.g. 2AgCl → 2Ag + Cl2
○ E.g. 2AgBr → 2Ag + Br2
Decomposition reactions are usually endothermic as they require energy to break the bonds
○ Decomposed through the application of heat, making it an endothermic
reaction
○ E.g. CaCO3 → CaO + CO2
■ Calcium carbonate → Calcium Oxide + Carbon Dioxide gas
● Electrolytic
○ Decomposition due to an electric current being passed through an aqueous
solution of a compound
■ Electrolytic cells are used for electrolytic decomposition
○ E.g.NaCl→Na+Cl
■ Sodium Chloride → Sodium + Chloride
○ E.g. 2H2O → 2H2 + O2
■ Water → Hydrogen gas + Oxygen gas
● Photo Decomposition
○ Decomposition due to use of light energy/photons
○ E.g. 2AgCl → 2Ag + Cl2
○ E.g. 2AgBr → 2Ag + Br2
Decomposition reactions are usually endothermic as they require energy to break the bonds
Types of Decomposition Reactions
96
New cards
Acid + Base → Salt + Water
When an Acid and Base react, the (OH-) and (H+) create water and neutralise each other, forming a salt
● This is called a neutralisation reaction, because the H+ in acids and OH- in bases react and produce water, and this brings the pH closer to 7 (since pH is based on concentrations of H+ or OH- ions)
● Different acids will produce different salts E.g. HCl + NaOH → NaCl + H20
Hydrochloric Acid + Sodium Hydroxide → Salt + Water
When an Acid and Base react, the (OH-) and (H+) create water and neutralise each other, forming a salt
● This is called a neutralisation reaction, because the H+ in acids and OH- in bases react and produce water, and this brings the pH closer to 7 (since pH is based on concentrations of H+ or OH- ions)
● Different acids will produce different salts E.g. HCl + NaOH → NaCl + H20
Hydrochloric Acid + Sodium Hydroxide → Salt + Water
Neutralisation reaction
97
New cards
Acid + Metal → Salt + Hydrogen gas
When acid reacts with a metal, hydrogen gas and a salt is produced
● Some metals react more rapidly than others i.e. magnesium
● Some metals require heat to begin the reaction
● Other metals such as gold will not react with weaker acids
E.g.Mg+H2S O4 →MgS04 + H2 E.g. Mg + 2HCl → MgCl2 + H2 E.g. Pb + H2SO4 → PbSO4 + H2
When acid reacts with a metal, hydrogen gas and a salt is produced
● Some metals react more rapidly than others i.e. magnesium
● Some metals require heat to begin the reaction
● Other metals such as gold will not react with weaker acids
E.g.Mg+H2S O4 →MgS04 + H2 E.g. Mg + 2HCl → MgCl2 + H2 E.g. Pb + H2SO4 → PbSO4 + H2
Acid and metal reaction
98
New cards
Metal Carbonate + Acid → Salt + Carbon Dioxide + Water
When an acid reacts with a metal carbonate or bicarbonate, a salt, carbon dioxide and water is produced
● Citric acid reacting with sodium bicarbonate produces the fizzy sensation of sherbet
E.g. 2HCl + Na2CO3 → CO2 + H2O + 2NaCl E.g. H2SO4 + CuCO3 → H2O + CO2 + CuSO4
When an acid reacts with a metal carbonate or bicarbonate, a salt, carbon dioxide and water is produced
● Citric acid reacting with sodium bicarbonate produces the fizzy sensation of sherbet
E.g. 2HCl + Na2CO3 → CO2 + H2O + 2NaCl E.g. H2SO4 + CuCO3 → H2O + CO2 + CuSO4
Metal Carbonate + Acid
99
New cards
Metal Oxide + Acid → Salt + Water
When a Metal Oxide and an Acid react, salt and water is produced
E.g.CuO+2HCl→CuCl2 +H2 O
When a Metal Oxide and an Acid react, salt and water is produced
E.g.CuO+2HCl→CuCl2 +H2 O
Metal Oxide + Acid
100
New cards
Things burning are indicative of reactions with oxygen, where flames (light, heat and sound energy) can be observed - these are known as combustion reactions, and are exothermic
● Other reactions with oxygen are corrosion reactions - these are much slower
Incomplete combustion (not enough oxygen)
Hydrocarbon + Oxygen → Carbon Monoxide + Carbon + Water
Complete Combustion
Hydrocarbon + Oxygen → Carbon Dioxide + Water
E.g. Complete combustion of methane C H 4 + 2 O 2 → C O 2 + 2 H 2 O
E.g. Incomplete combustion of ethane C2H6 +2O2 →CO+C+3H2 O
● Other reactions with oxygen are corrosion reactions - these are much slower
Incomplete combustion (not enough oxygen)
Hydrocarbon + Oxygen → Carbon Monoxide + Carbon + Water
Complete Combustion
Hydrocarbon + Oxygen → Carbon Dioxide + Water
E.g. Complete combustion of methane C H 4 + 2 O 2 → C O 2 + 2 H 2 O
E.g. Incomplete combustion of ethane C2H6 +2O2 →CO+C+3H2 O
Combustion reaction