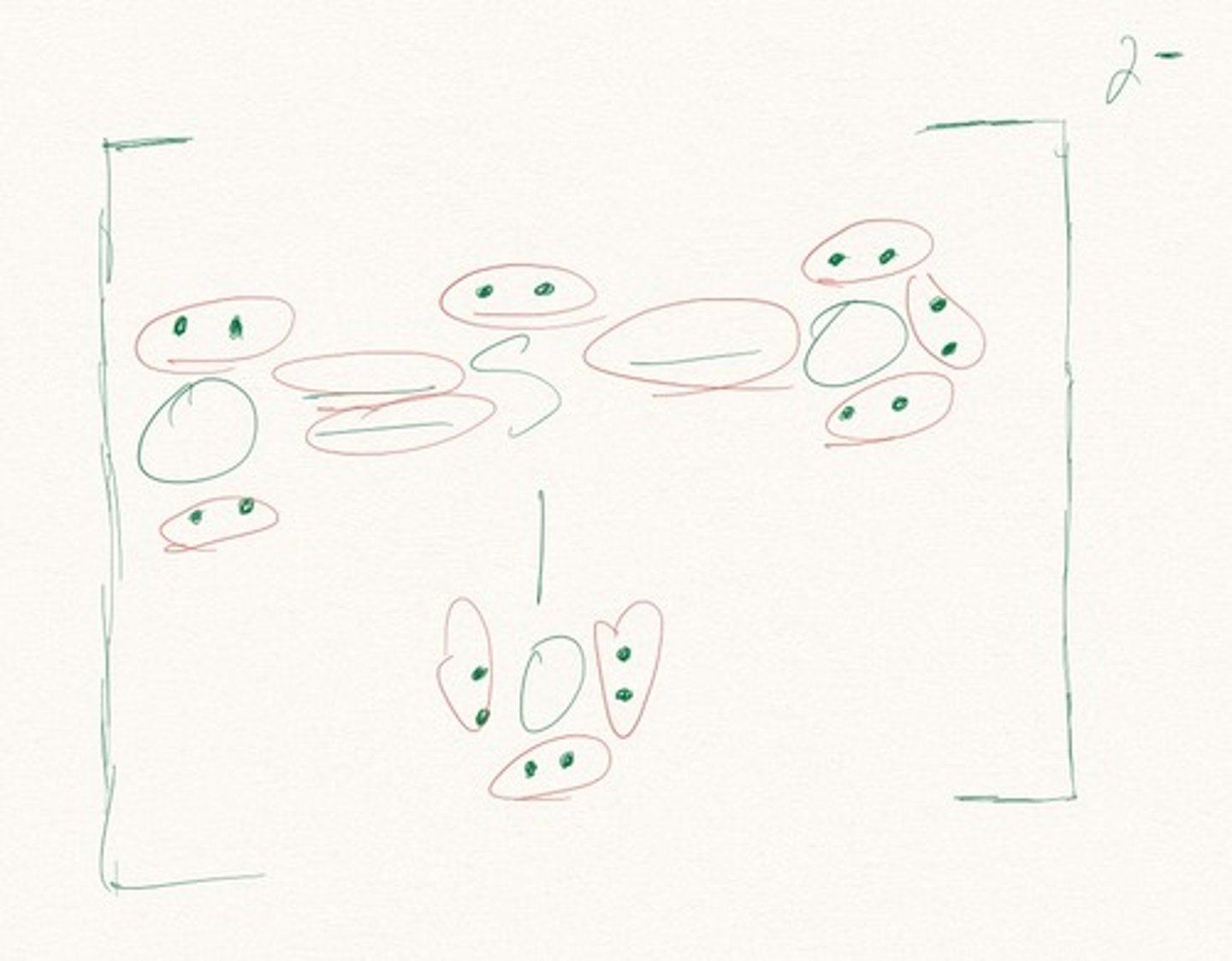
Lewis Dot Structure, Lewis Dot Structures
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Which elements make expanded octets? What are they commonly combined with?
P, S, As, Se, Br, I
Commonly combined with F or Cl
Which group of atoms don't double bond (VERY IMPORTANT REMEMBER)
Halogens (7A)
Which elements can never be the central atoms?
Hydrogen and Flourine
How many bonds does can Boron make max?
3 bonds
How many bonds does can Beryllium make max?
2 bonds
what are resonant structures?
structures that can be written in more than one way
What do you do when the molecule is an ion
put brackets around the structure and indicate how many electrons are to be gained/lost
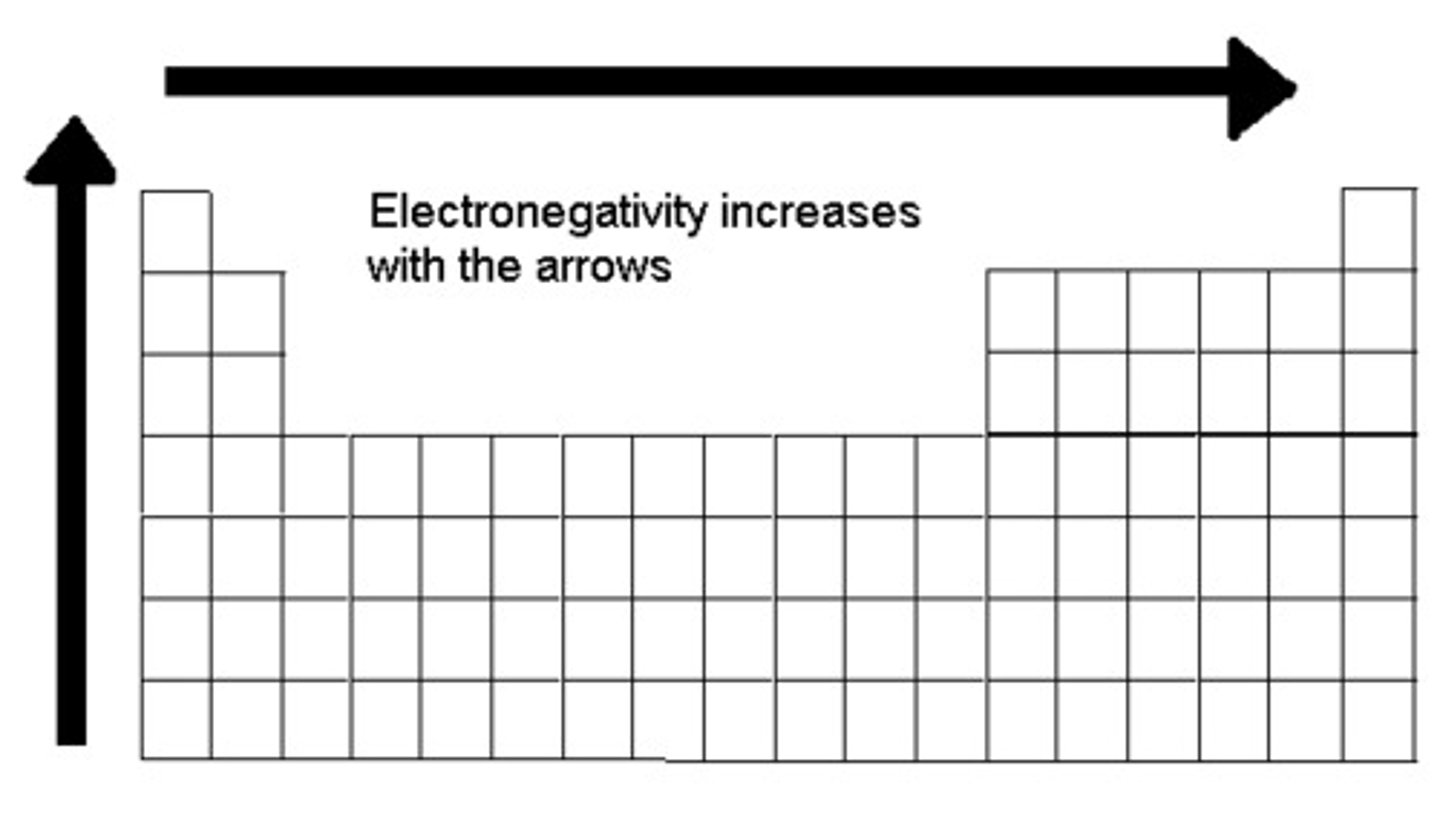
what is the trend for electronegativity?
what makes a molecule polar vs non polar?
polar: asymmetrical electronegativity and geometry
non polar: symmetrical electronegativity and geometry
what does a linear molecule look like?
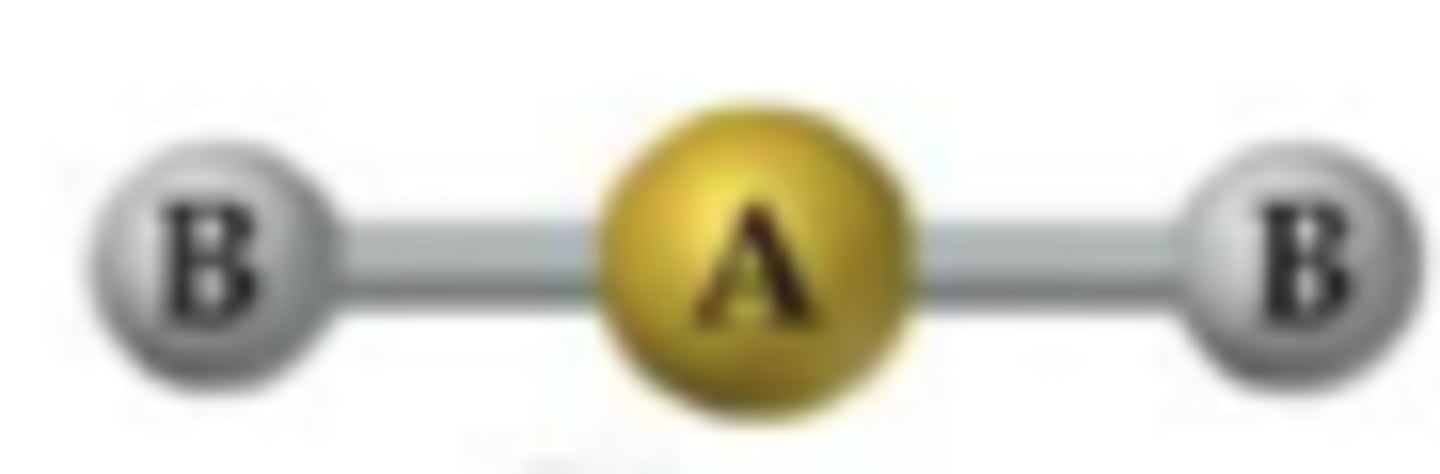
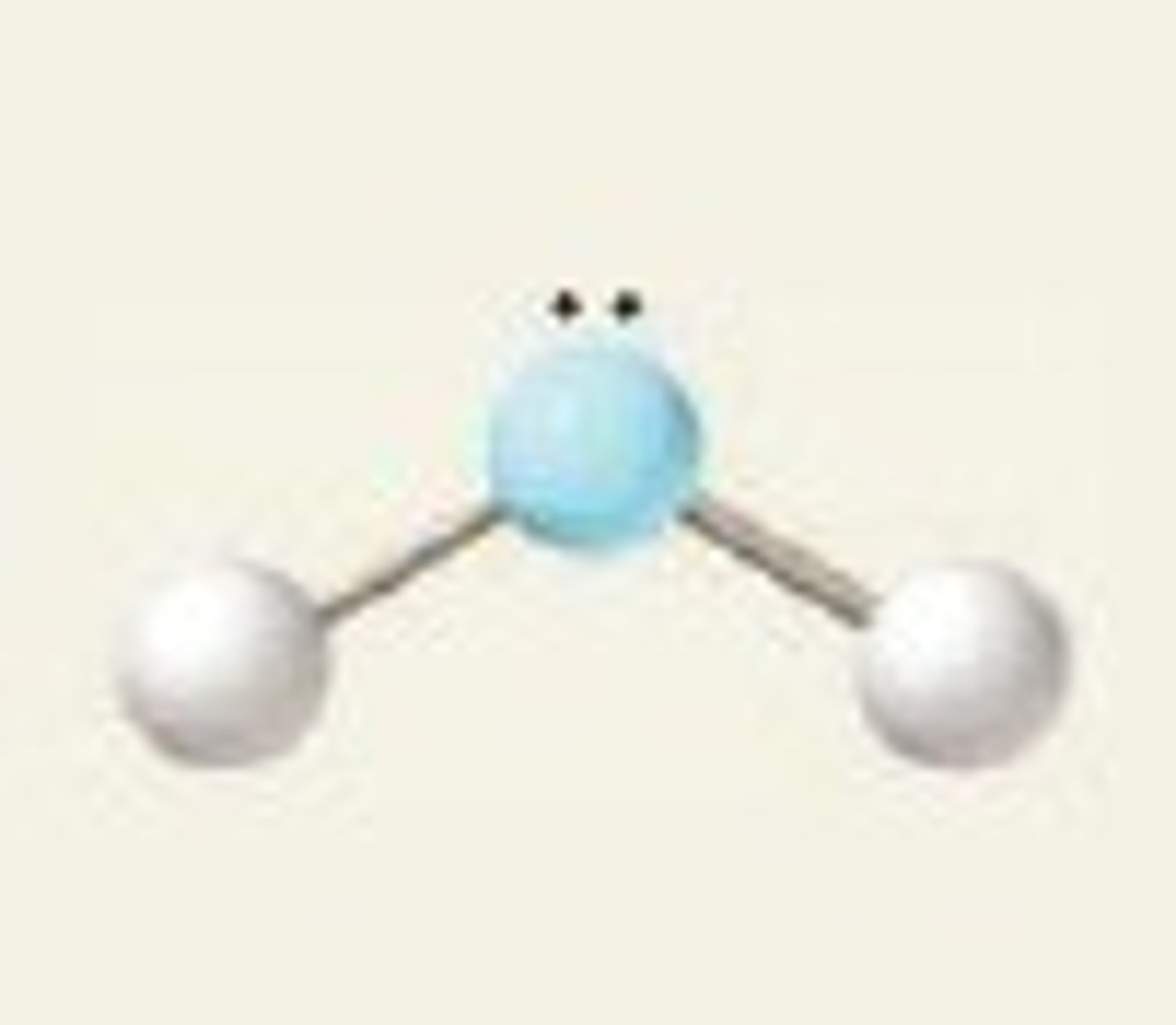
what does a v shaped or bent molecule look like?
How many BONDING PAIRS does PCl3 has?
4
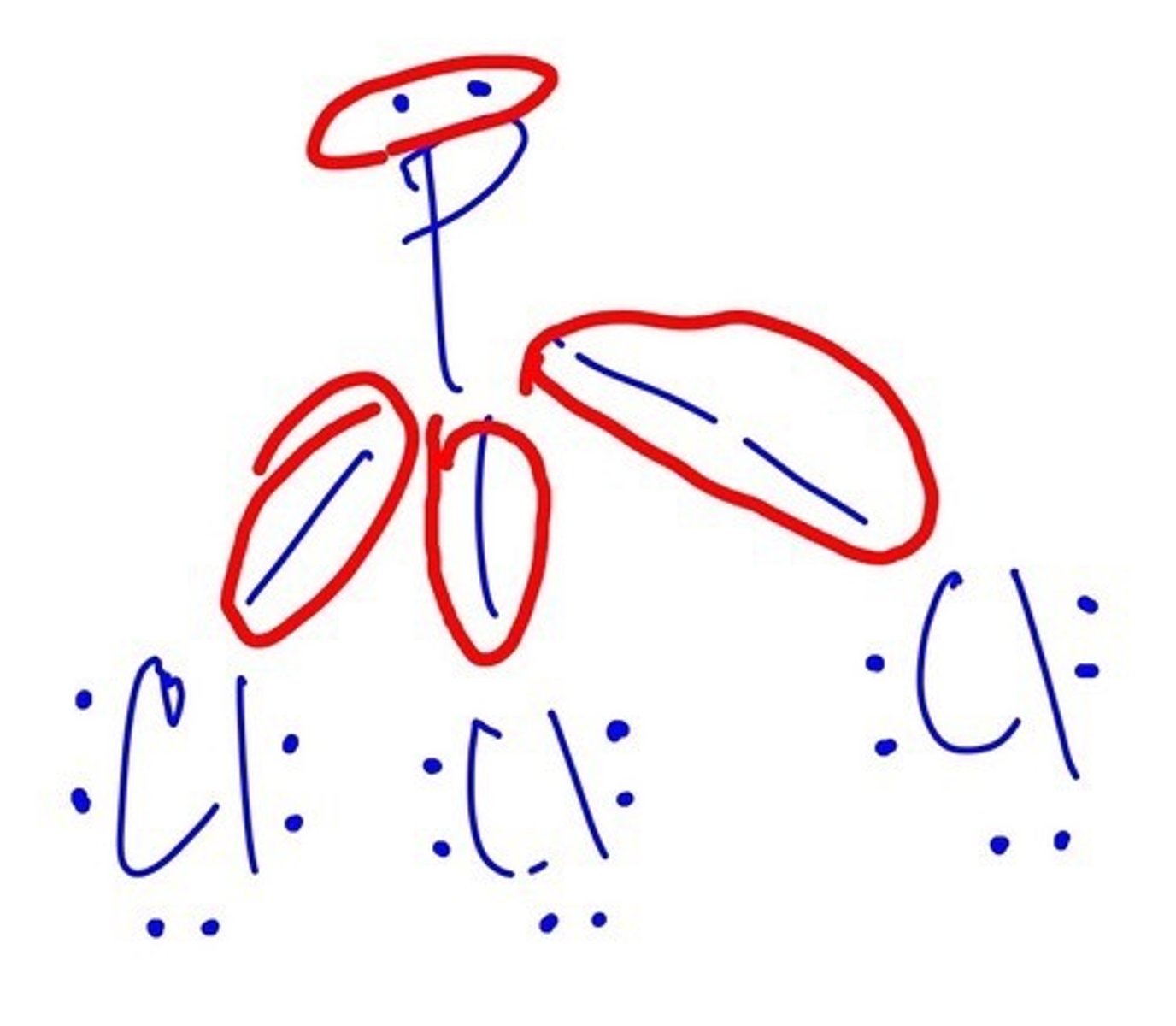
How many VALENCE PAIRS does PCl3 has?
10
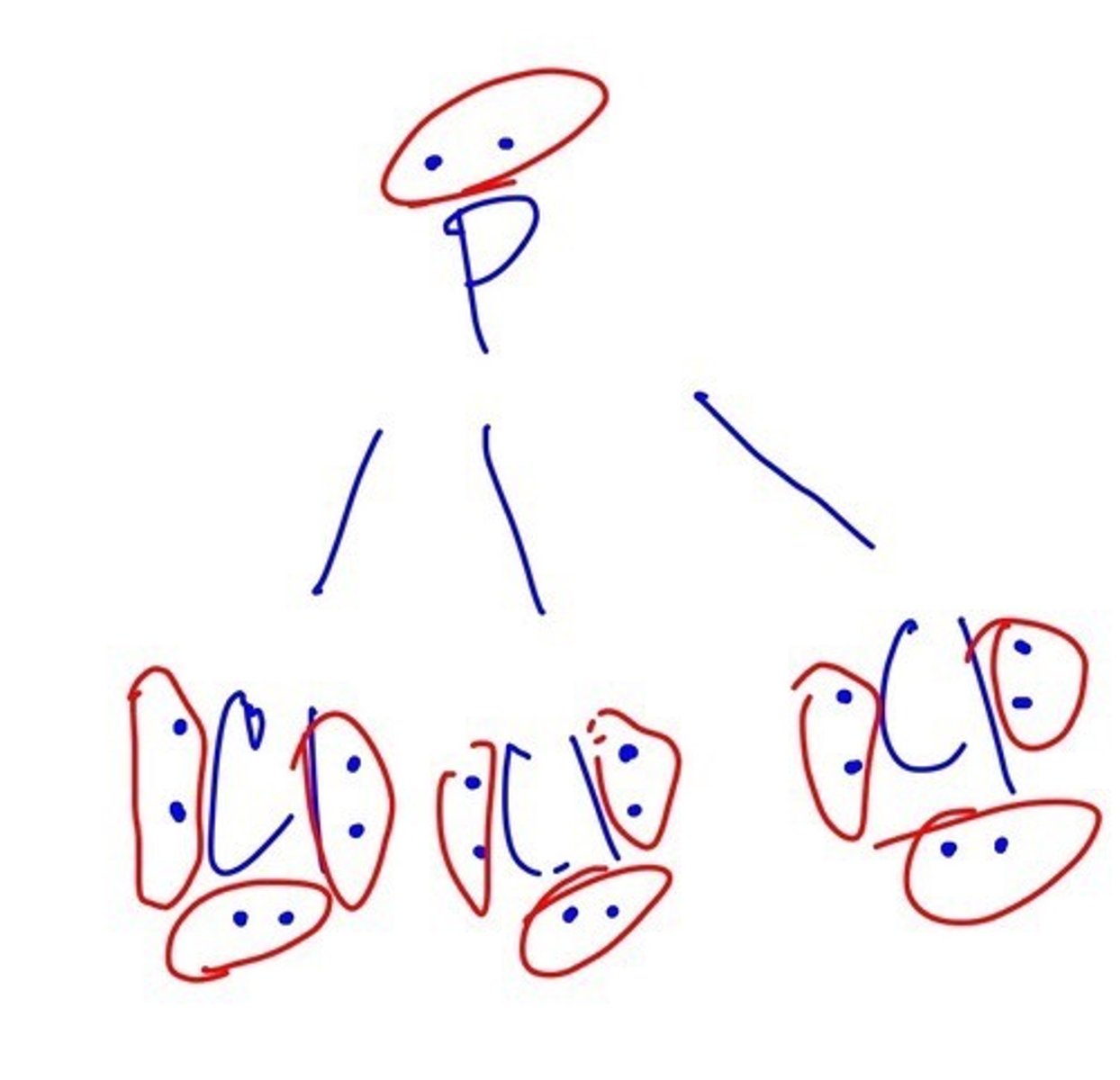
How many ELECTRON PAIRS does PCL3 has?
13
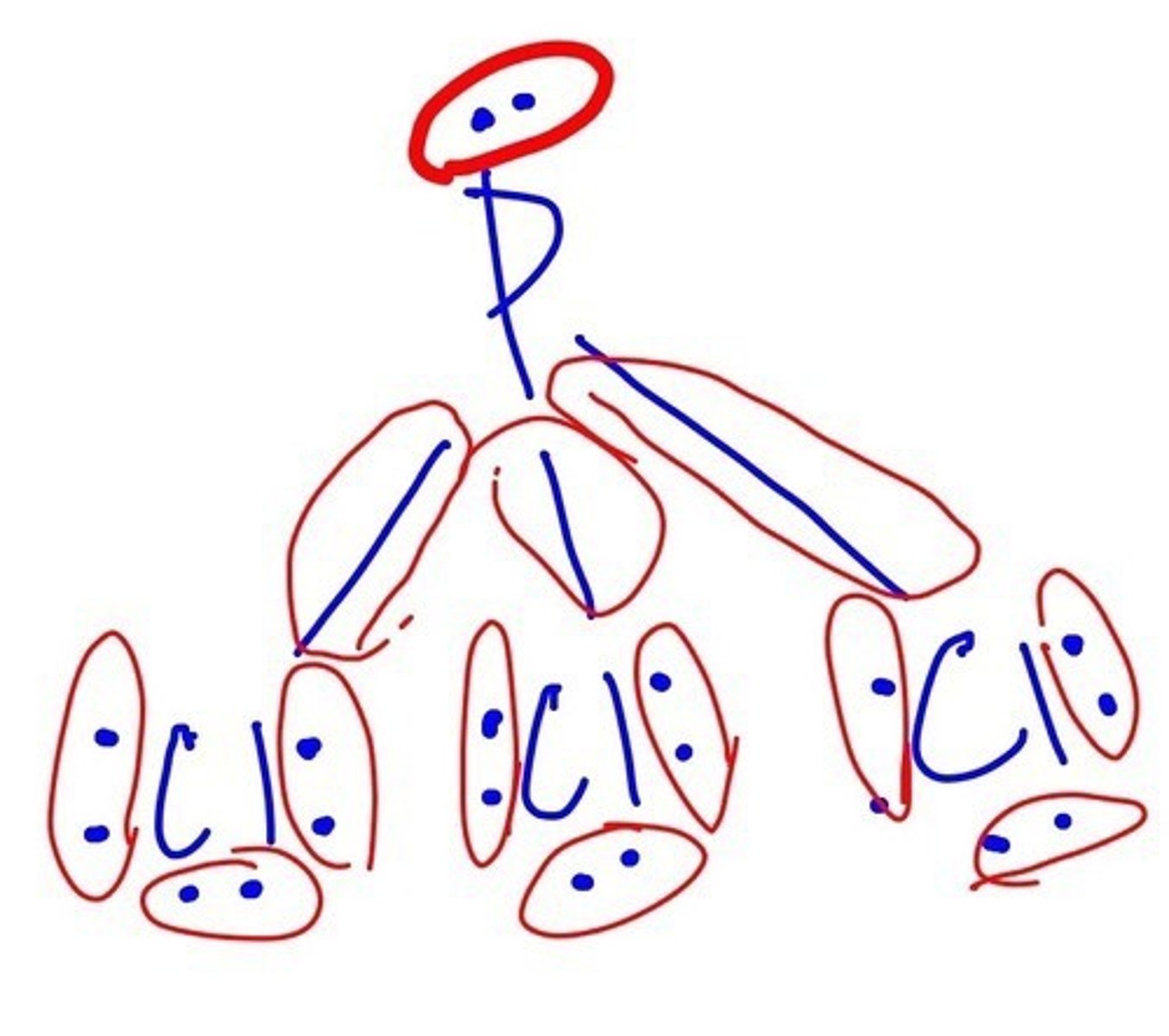
How many electrons are in PCl3?
26 electrons
What happens when bond electrons?
You lose 1 electron from each element and there's 2 electrons per bond
Draw the Lewis Dot Structure for O3? What is the total number of valence ELECTRONS?
18 valence electrons
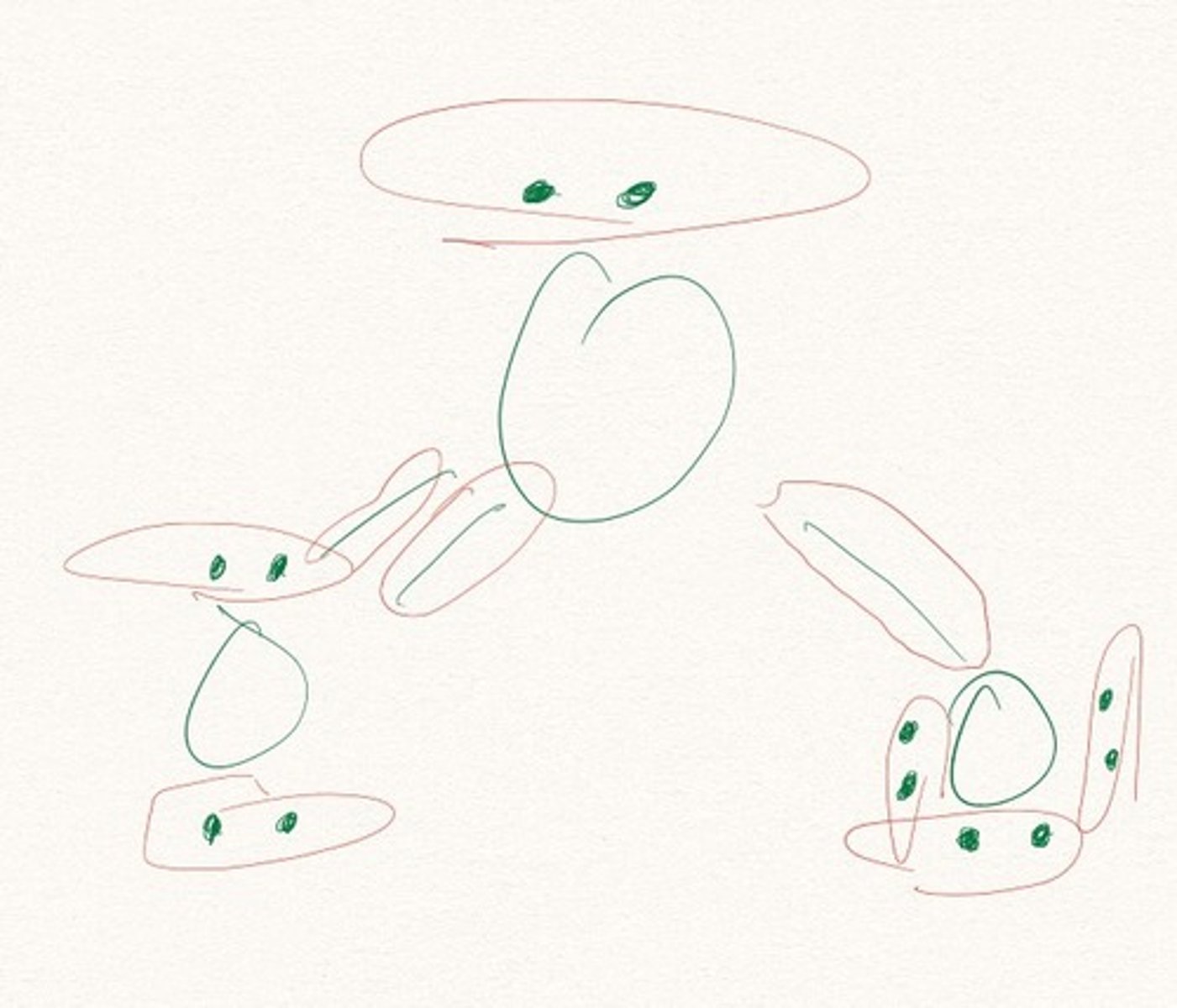
Draw the Lewis Dot Structure for SO3^2-? What is the total number of valence ELECTRONS?
26 valence electrons