exam 2- biochemistry
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
what is the difference between saturated, polyunsaturated, unsaturated, cis and trans fats
saturated: no kinks and only single bonds
polyunsaturated: multiple double bonds
unsaturated: 1 double bond
cis fat: is a unsaturated fatty acid with 2H in the same positon (most common and found in nature)
trans fat: an unsaturated fatty acid with 2H in opposite positions (artifical)
what part of a fatty acid is polar, and which is nonpolar
The carboxylic acids are the polar head, and the hydrocarbon chain is the nonpolar tail that faces inside.
what are the factors that contribute to the melting point of a fatty acid
1) length of the nonpolar tail, the longer the more tightly packed and the more stacking and van derwal bonds
2) number of double bonds. the more there are the lower the melting point bc the kinks keep them from laying flat together and keeps it liquid (think sunflower oil at room temp which is polyunsaturated)
what do the numbers for a saturated fatty acid tell us
first number is the total amount of carbons present and the second is the total number of double bonds. so it should always be a number to 0 (12:0 lauric acid)
what is the difference between the omega and delta system when identifying unsaturated fatty acids
omega: first number is the amount of carbons, second number is the amount of double bonds, and third number is the carbon that the double is located starting from the NONPOLAR END/hydrocarbon. (ex: 16:1n-7 is palmitoleic acid)
delta: is the same thing indicates the carbon from the POLAR END/carboxylic!
what are other names for fat and what is the structure. what type of bonds hold it together
triglycerides/triacylglycerols
(3 esterifed fatty acids attached to a common backbone. the 3 FA can be different)
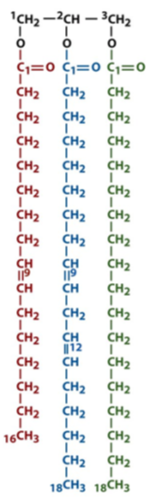
what is the storage form of a fatty acid and where is it stored
triglyceride/triacylglycerol/fat which is stored into adipocyte cells
how much more metabolic energy do fats produce compared to glycogen
6x more
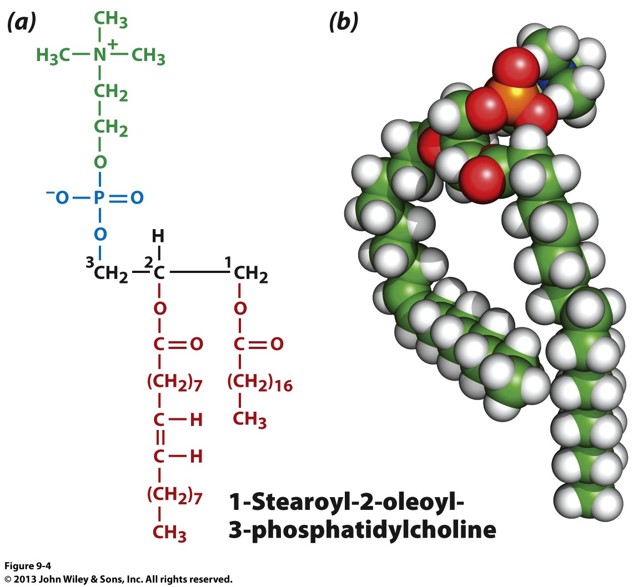
what is the structure of a glycerophospholipid?
3 carbon backbone with 2 FA chains attached to carbons 1 and 2. Carbon 3 has a phosphate and functional group (VERY COMMON IN MEMBRANE) 1 HEAD 2 TAIL
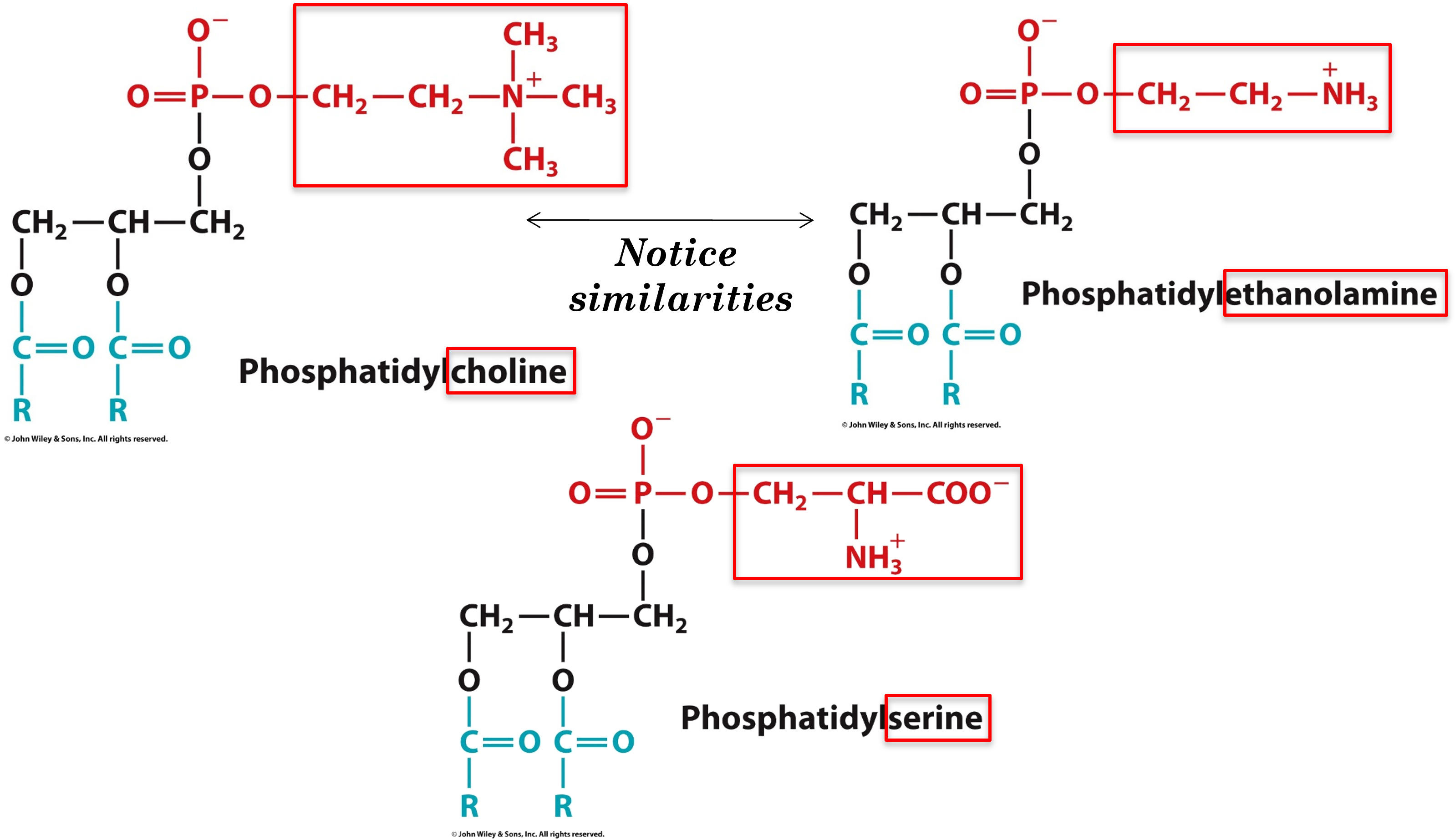
what are the 3 most common head groups that could attach to glycerophospholipids phosphate group
choline, ethanolamine, serine
what are phospholipases
there are enzymes that can cut a glycerophospholipid in specific spots
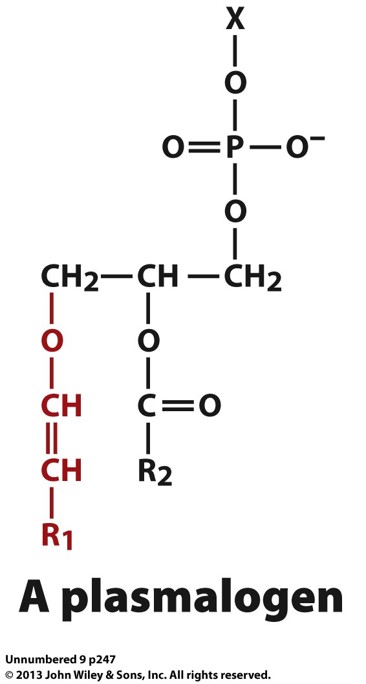
we know that glycerophosploids have fatty acids that usually attach on the first 2 carbons of the 3 carbons it contains. theses carbons usually have Ester bonds. what does it become when carbon 1 is replaced with an ether bond?
it becomes a modified version called a plasmalogen
what is the backbone of a sphingolipid called? and what commonly attaches to it?
sphingosine. ceramide attaching to the amine group OR a phosphocholine attaching to first carbon
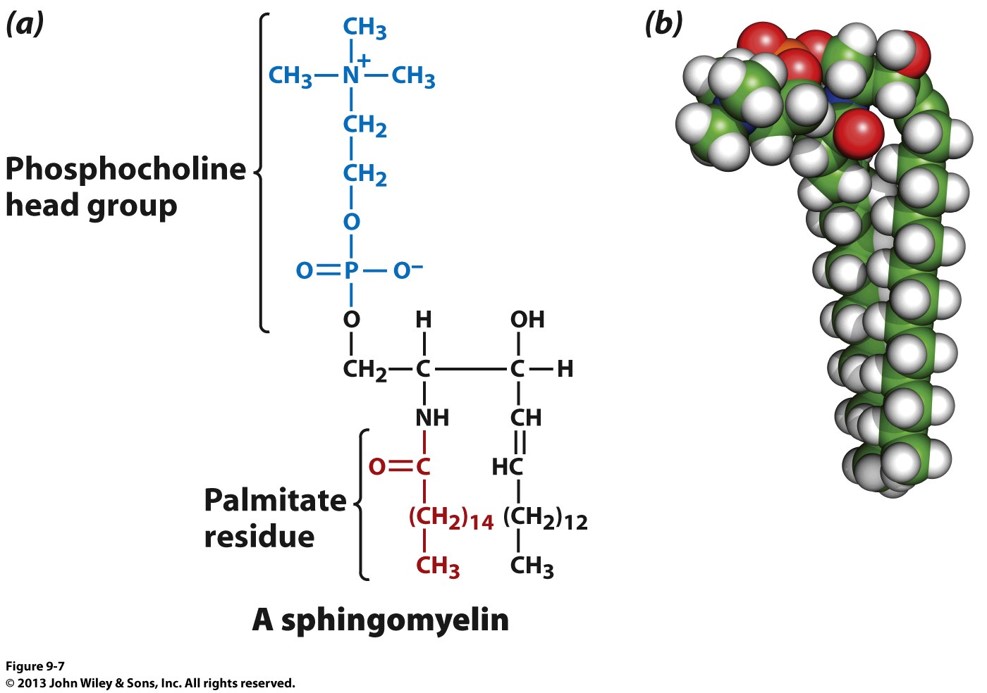
what is this? which is the backbone and whats its name
a type of sphingolipid called a sphingomyelins . the backbone is in black and the group in blue makes it the sphingomylein. ONE HEAD 2 TAILS
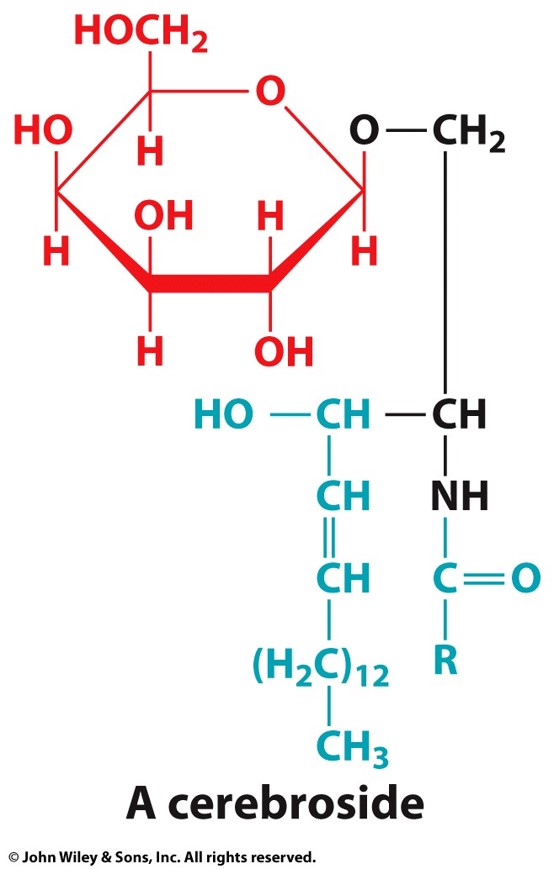
what is a sphingolipid that has a monosaccharide as a head group
a cerebroside
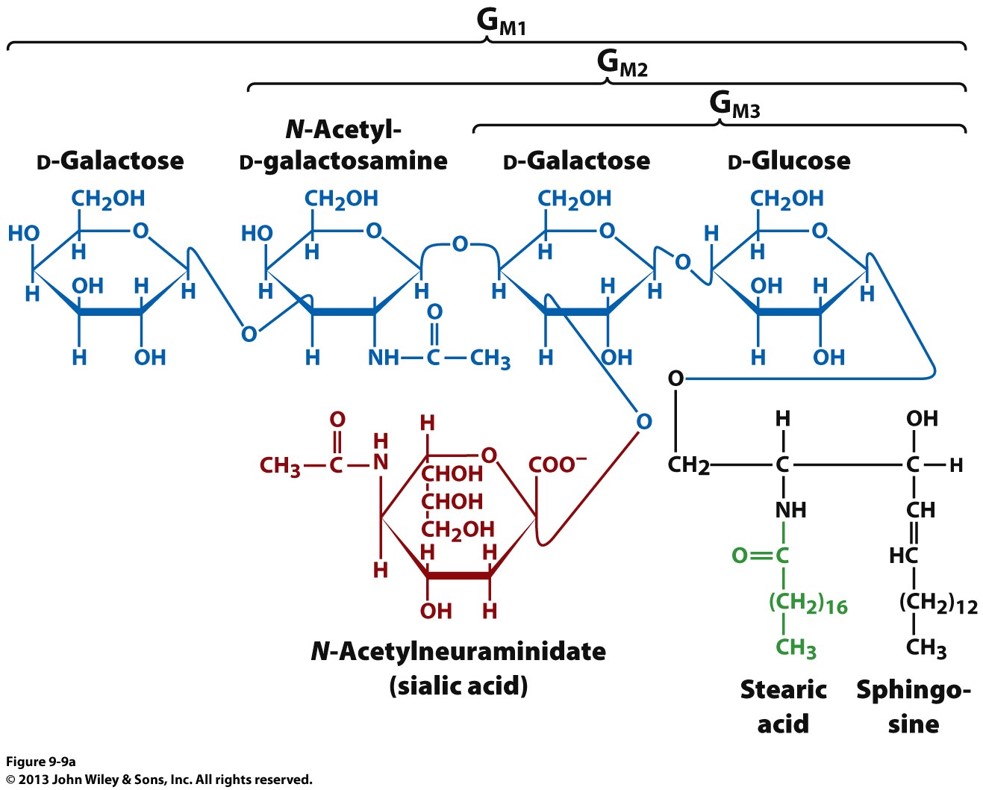
what is a ganglioside?
a sphingolipid with oligosaccharides as head groups. black is the backbone (sphingosine), blue is the oligosaccharides, green is fatty acid
what is the most abundant steriod and in plasma membranes? What else is it used for? what makes it more rigid than other lipids
cholesterol /isoprenoids (another lipid) and the fused ring makes it stable. its used in hormones and vitamin synthesis
What’s the difference between a liposome and a cell membrane? what are their membranes made out of and why does that benefir them
• Liposomes:
• Spherical structure with one lipid bilayer surrounding water core
• formed by glycerophospholipids or sphingomyelins (remember, they have a square structure so it seals the vesicle)
• Lipid Bilayers (cell membrane):
• Two layers of lipids, Hydrophobic tails inward, hydrophilic heads toward water
• It also has cholesterol and proteins embedded in its membrane, which makes it semi permeable
What are micelles and how do they work?
they are individual molecules that grouped together to trap nonpolar molecules like oils inside themselves. They have a hydrophilic head and a hydrophobic tail. when there is oil floating around in a aqueous solution, the tails will attach to the oil and the heads would stay in contact with the water surrounding the oil.
what are factros of lipi bilayers
-its fluid and the heads can move from left to right (fast process called lateral diffusion), sometimes across (slow process called transverse diffusion). The hydrocarbon tails wave
-also is asymmetical, different lipids are found in each leaflet
how do the lipid bilayers/cellular membrane maintain its fluidity
its temperature dependent.
- if the temp is low, make new shorten lipids and replace long with shorter (less carbons) and increase the number of double bonds(or unsaturation/kinks), so it doesnt pack as tightly, which lowers the mp and keep the fluidity
what are the 3 ways a protein can be linked to a membrane
1) integral membrane proteins (the residues imbedded in the membrane are nonpolar and hydrophobic, since the inside of the membrane is)
2) Peripheral membrane proteins
3) Lipid linked proteins
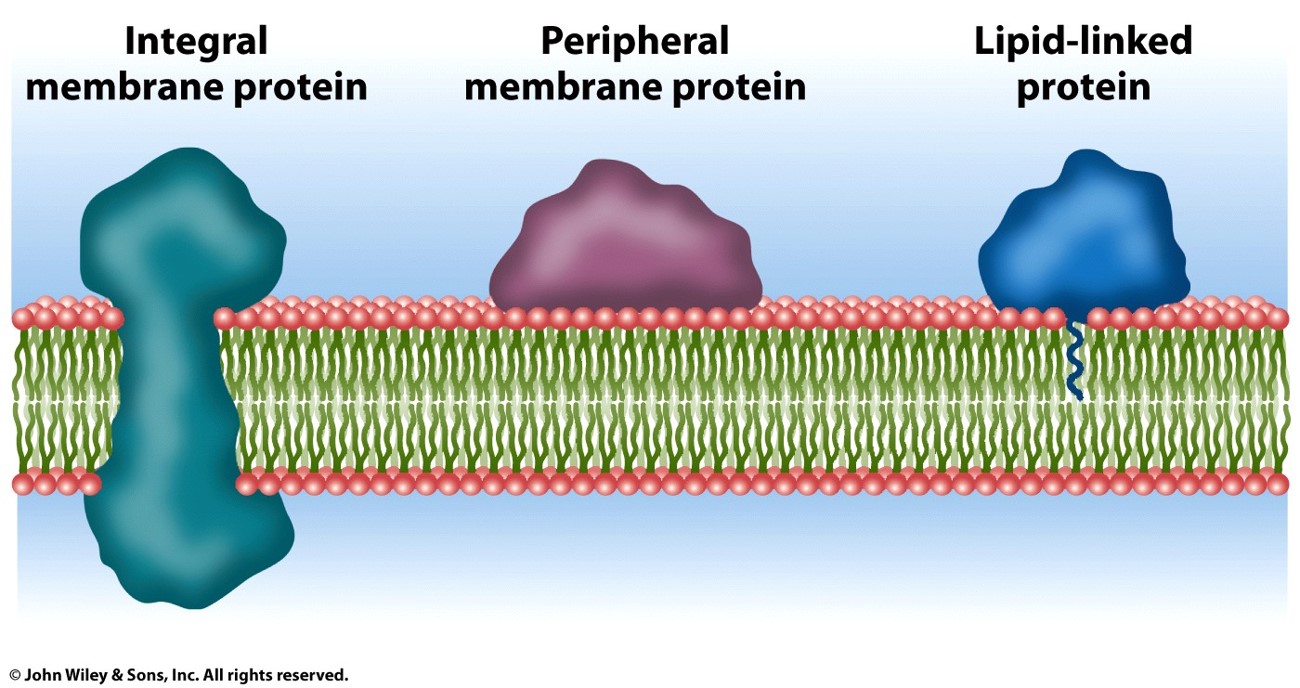
What’s the difference between the three proteins that could be embedded in a cell membrane?
Integral membrane proteins are embedded in the lipid bilayer and function in processes like transport, acting as channels or receptors (has both hydrophilic and hydrophobic parts)
• Peripheral membrane proteins are attached to the surface of the membrane and assist in signaling, maintaining cell shape, or supporting other membrane proteins.(hydrophilic)
• Lipid-linked proteins are anchored to the membrane by lipid molecules and play roles in signaling and membrane structure. (Hydrophilic attached to head)
what is another name for plasma membrane or lipid bilayer
fluid mosaic model (proteins attached flow through a sea of lipids)
what is inner vs outter leaflet and what do the breakdowns tell us
it means inside verses outside of the cell, and they contain different types of lipids in varying amounts depending
ester vs ether
what are the 2 most common lipids found inside the membrane? (inner leaflet)
sphingololipuds and glycerolphospholids.
why are the most 2 common types of lipids the most common
they have 1 head and 2 tails so it gives them a square shape and they fit next to each other and create a perfect seal
what lipidS ARE not good for membreanes and why
fatty acids AND triacylglycerol’s because fatty are 1H1T and tri is 1H3T forming a triangle shape and leaving gaps.
What is simple diffusion?
Movement from high to low concentration. No assistance is needed and only small nonpolar molecules can cross so its a nonmediated transport (gas, hormones, steroids)
What is mediated transport and its types, and whats another name for the one what starts with a p
Transport for big polar molecules
1) passive mediated/facilitated diffusion: high to low
2) active transport: low to high and needs energy like ATP since its against the gradiant
What are the 4 passive mediated transport protiens
Ionophores, porins, aquaporins, ion channels
What are the types of ionophores (very specific proteins). whats an example
1) channel forming: is a pore for many ions to past through quickly
2) carrier: transports one ion at a time Very slow (Valinomycin for K+)
What are porins? what proteins are they made of and their insides and what determines what can pass
A membrane beta sheet protein with a aqueous passage. The residues that make up the passage determines what can pass.
usually for carbs and big molecules
Whats a protein that only moves water in and out the membrane? What type of structure is it made of?
Aquaporins made of alpha helices, 4 subunits
What are ion channels, what type of transport and wht is necessary to pass
Very specific protiens only letting specific ions through. It must match the selectivity filter to be able to pass, and that is caused by the side chains. They are gated channels but is passive transport, so high to low concentration
Whats an example of an ion gated channel
A nerve impulse, allowing sodium and potassium to cross in a controlled way.
how do transport proteins work? whats an example
They literally change the shape of the protein to when the molecule that needs to be moved is captured. The shape changes again to be released. (GLUT1 for ex)
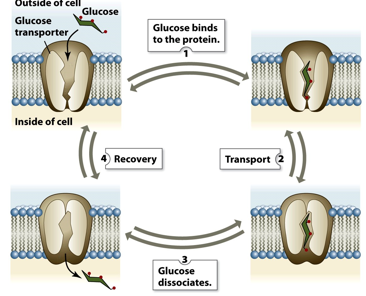
What are the types of mediated transport movements?
Uniport- 1 thing at a time
Symport- 2 in the same direction
Antiport- 2 in the opposite direction
Tell me about the P type ATPase active transportor and the most common one
They undergo phosphorylation when they transport cations like Na and K (remember low to high). Most common is NAK ATPASE which is an antiport, Na out K in.
How does na-k-apase work (which is a p type atpase bc of phosphorylation)
It takes seven steps, three sodium ions out two potassium ions in, and this cost 1 ATP.
Bc of atp hydrolysis if the pocket is facing inside the cell then sodium can go into the protein, but if the pocket is facing outside of the cell, then potassium can go into the protein (2 types of conformations)
But at the end sodium will be outside and potassium will be inside.
What is ca-atpase
A p type active transportor that transfers 2ca out 3 protons in by atp hydrolysis
What are ABC transporters
They are active transporters, which requires ATP and it has two confirmations of opened toward the outside of the cell were open toward the inside of the cell.
What is phosphorylated it’s one structure when does dephosphorylated it’s another structure
It works as defense for cells because it moves objects out from low to high concentration
So for instance, if the transporter is open and a foreign object enters, it will do a confirmational change and turn 90° to kick that particle back out of the cell.
What is the difference between secondary and primary transport?
ATP is used directly in primary transport (p types which need atp to change the structure of protein) but indirectly in secondary transport.
what are the 3 ways to increase the rate of a chemical reaction
1) increase temperature/adding energy
2) increase concentration of the reactants
3) add a catalyst/enzyme
what catalyst is NOT a protein
Ribozymes which are RNA molecules
what are the 4 ways enzymes different than an ordinary chemical catalyst?
1) higher reaction rates
2) they need mild conditions to function (like the bodies conditons)
3) they function with specific substrates and rarely produce biproducts
4) can be controlled and they can change based on its environment
how are enzymes usually named
theyre named after the reaction they catalyze. the second part of the name explains what is does (decarboxylase for example is an enzyme removing carboxyl groups
where do substrates bind?
to the active site of the enzyme which activates them
how does an enzyme even work? use NaCl
When an enzyme is added to a solution containing 2 reactants/substrates like Na and Cl, the substrates are then captured and attach to the active site of the enzyme. Once attached, the reaction will happen in the enzyme in a more ideal environment, making it happen faster. The now product will detach from the enzyme since its now NaCl and is not a fit for the active site anymore.
what is used when an enzyme may need some help and what are the 2 types
cofactors such as coenzymes (organic molecules) or metal ions
what is the most unstable and the energy barrier that determines the rate of a reaction?
peak of the reaction or the transition state, very high in free energy
true or false: the lower the activation energy the more difficult it is for a reaction to happen
false. the higher the activation energy(change in G), the harder it is for the reaction to proceed
what do enzymes do for the activation energy?
it lowers the delta G making the reaction happen faster. the enzymes hold the substrates/reactants into place in the active site, since stabilizes them to react more efficiently. without the enzymes, the reactants/substrates will react but it may take more energy for them to since they need to get in the proper position etc
what do enzymes do to the free energy of the reactant and products?
nothing. it only decreases the activation energy at the transition state
what does free energy look like in a spontaneous reaction when it comes to the reactants and products?
the reactants have more free energy than the products. THE REACTION RELEASES ENERGY AS IT PROGRESSES. SO FREE ENERGY IS LESS THAN 0
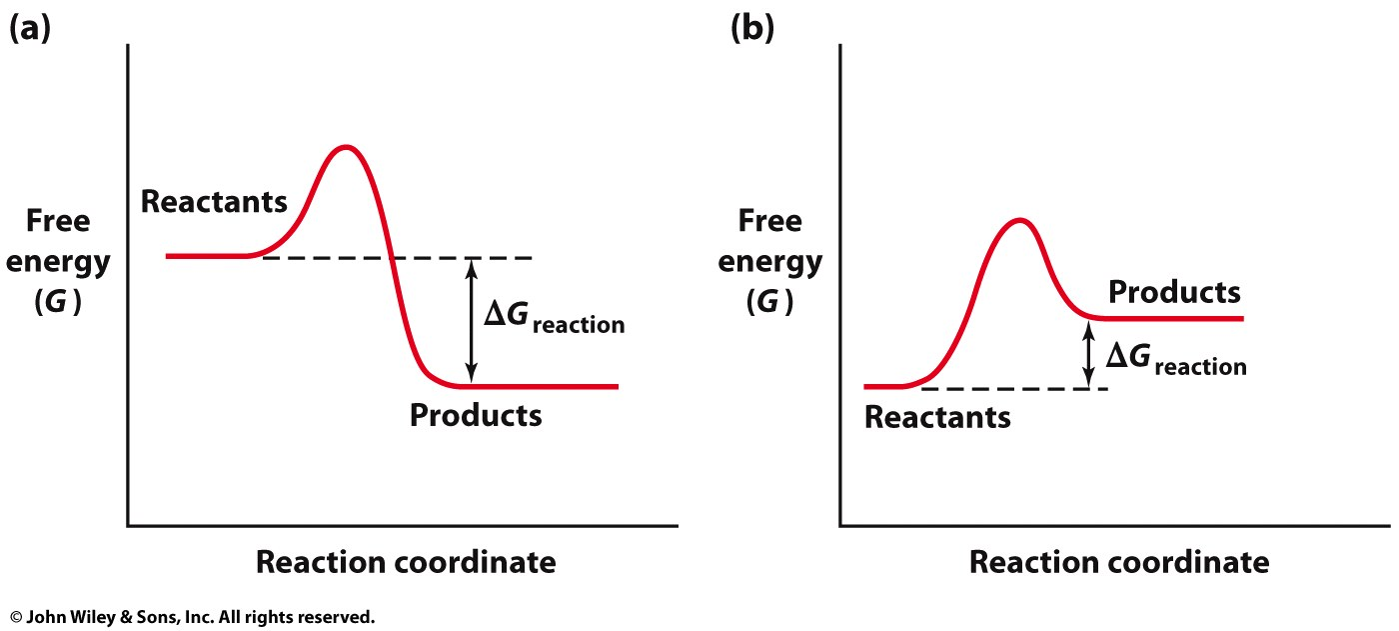
What does free energy look like in a non-spontaneous reaction?
the reaction absorbs energy to produce the product. so free energy is greater than 0.
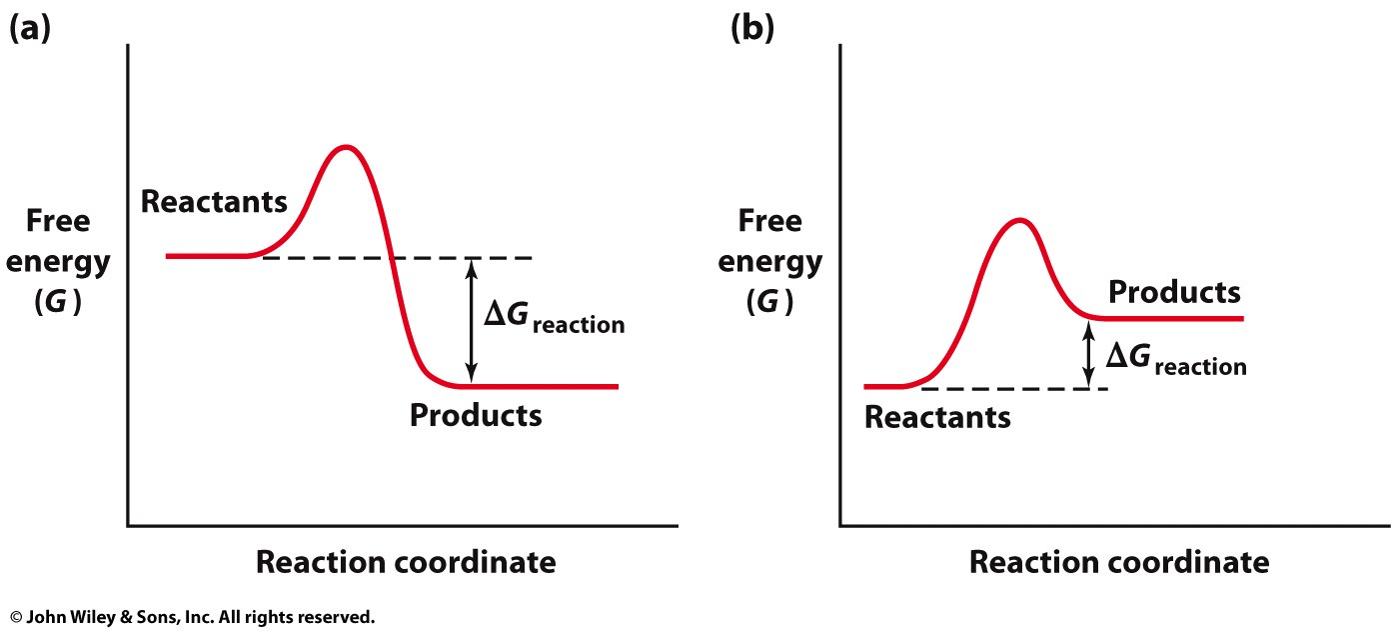
in a 2 step reaction, what is the rate determining step and why
the step that has the higher activation energy hump is the rate determining step because it can slow down the entire process even if the first step had a lower activation energy.
what part of a reaction is the most unstable with the highest energy?
the transition state
what is the most common mechanism that enzymes use to lower the activaation energy?
they do proton transfer by giving away protons (acid), or accepting protons (base) from the substrate that attaches to it. do this stabilizes the substrates/reactants more so they can form the product
what does proton transfer do for an reaction and why does it help
Enzymes can act as acids or bases by donating or accepting protons (H⁺) during a reaction. As an acid, an enzyme donates a proton to stabilize negatively charged intermediates during the transition phase where there’s usually a lot of activation energy. As a base, the enzyme accepts a proton to activate a substrate or make it more reactive. Proton transfer helps stabilize the transition state by neutralizing charges, making the reaction intermediates less unstable.enzymes can act differently depending on the step theyre at
how does ph affect enzymes
enzymes work best a certain ph, usually 7. changes in ph can mess with the side chains of the active site
what do temporary covalent bonds do for enzymes, and what is the nuc an elec
they stabilize the substrate (which is the electrophile) and the enzyme (which is the nucleophile) which reduces the AE.
what are the 3 ways that metal cofactors help a speed a reaction up
Binding to Substrates: Metal ions help orient substrates properly for the reaction.
Mediating Redox Reactions: Metal ions change their charge (oxidation state), allowing them to accept or donate electrons, which is essential in reactions where electrons are transferred.
Electrostatic Shielding/Neutralization: Metal ions stabilize or neutralize negative charges on molecules, making reactions easier.
why may an enzyme bind to a substrate during transition (high energy state) instead of before or after?
the enzyme can change the shape of the substrate/reactant by making it into the shape it needs to be for the transition phase more quickly and easily
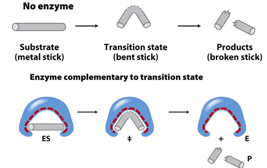
what do lysozymes do and what are the 2 amino acids that make up its active sites
they cut at the glycosidic bonds (curvy oxygens) and ASP 52 and GLU 35
what does serine proteases do, and what proteins make up their active sites (SHA)
they cleave peptide bonds in proteins, Ser, His, Asp